Preparation method of valienamine from acarbose and/or acarbose derivatives using trifluoroacetic acid
A technology of acarbose and trifluoroacetic acid, which is applied in the field of production of villetamide, can solve the problems of non-commercialization and production difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
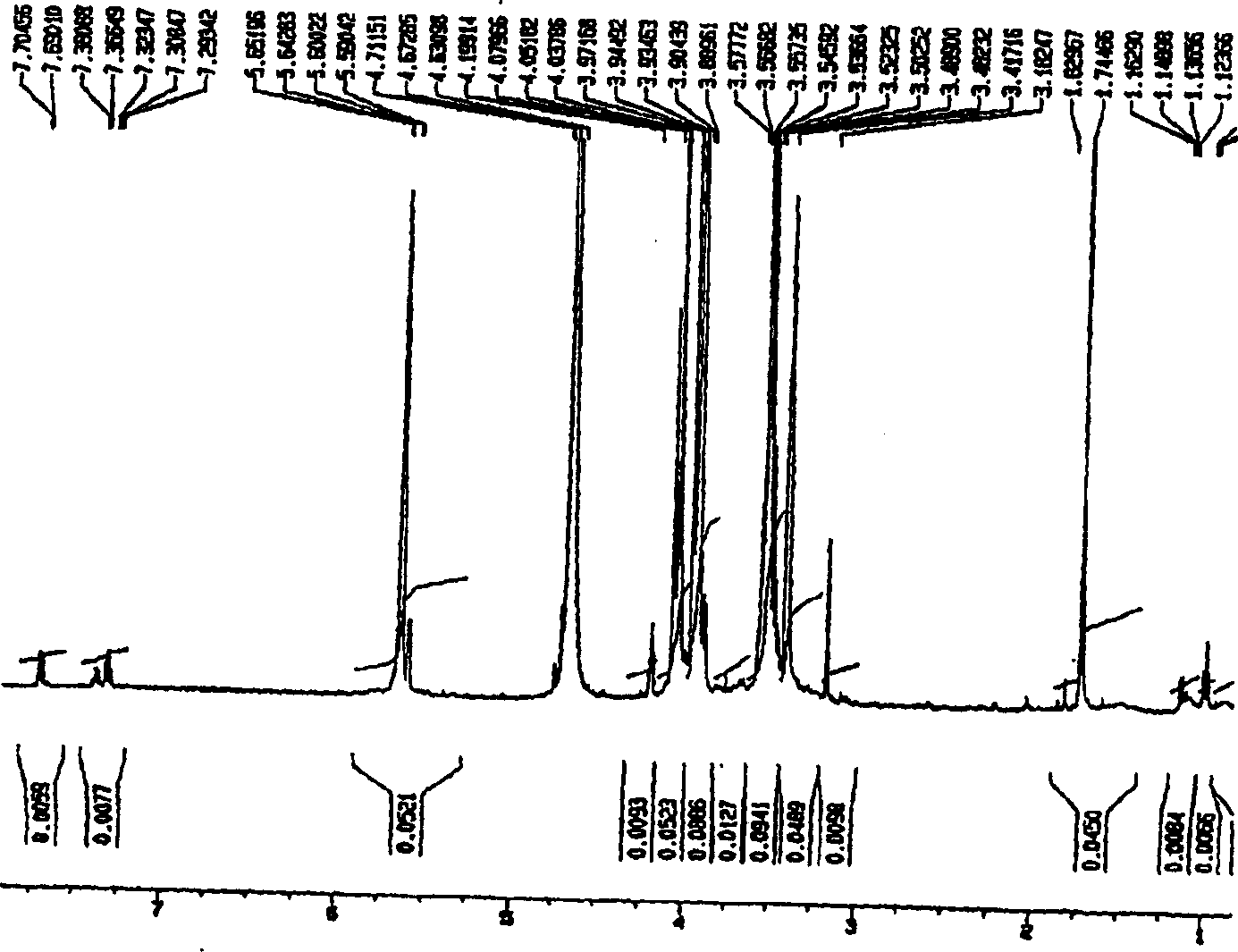
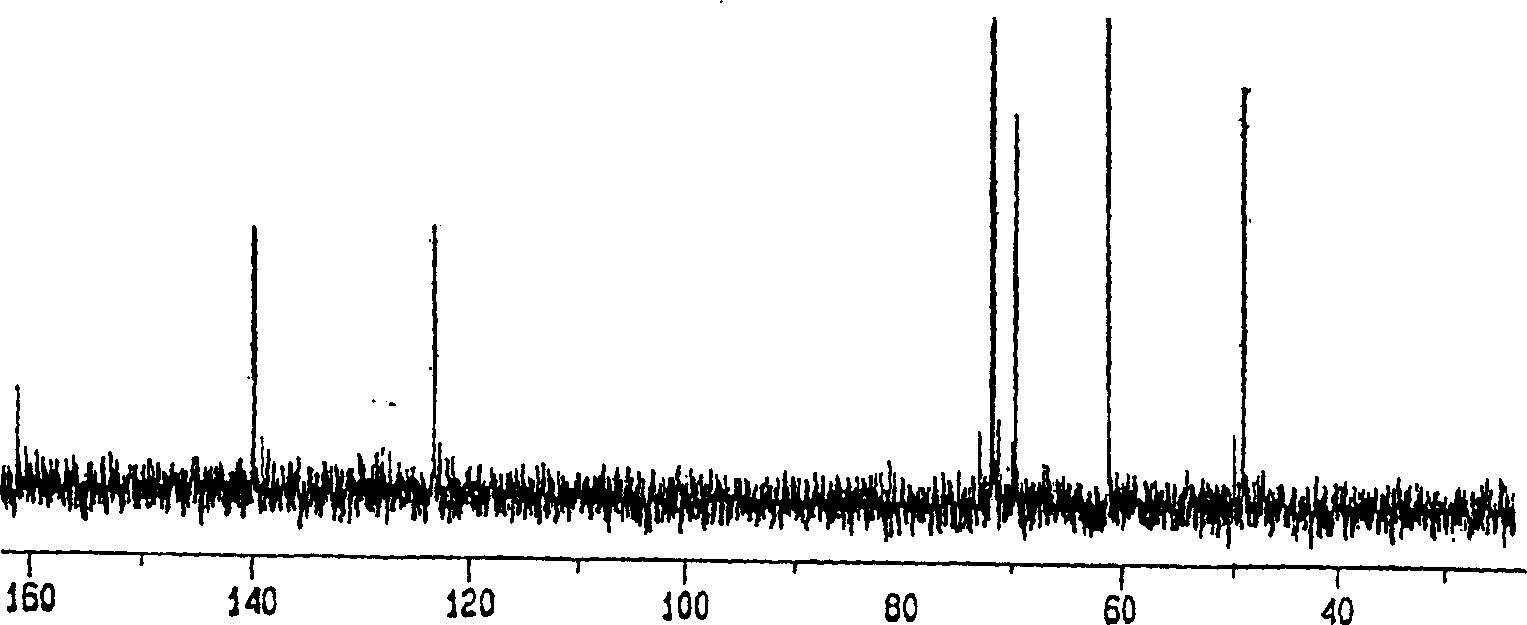
[0029] Embodiment 1: the method that uses TFA to produce visleytamide
[0030] Put 10 g of pure acarbose into 10% TFA solution to make the final concentration 5%. At a reaction temperature of 100° C., the system was allowed to react for 12 hours or more, followed by removal of TFA and water. Purification was then carried out by using an ion exchange resin to obtain 2.02 g of villetamide.
Embodiment 2
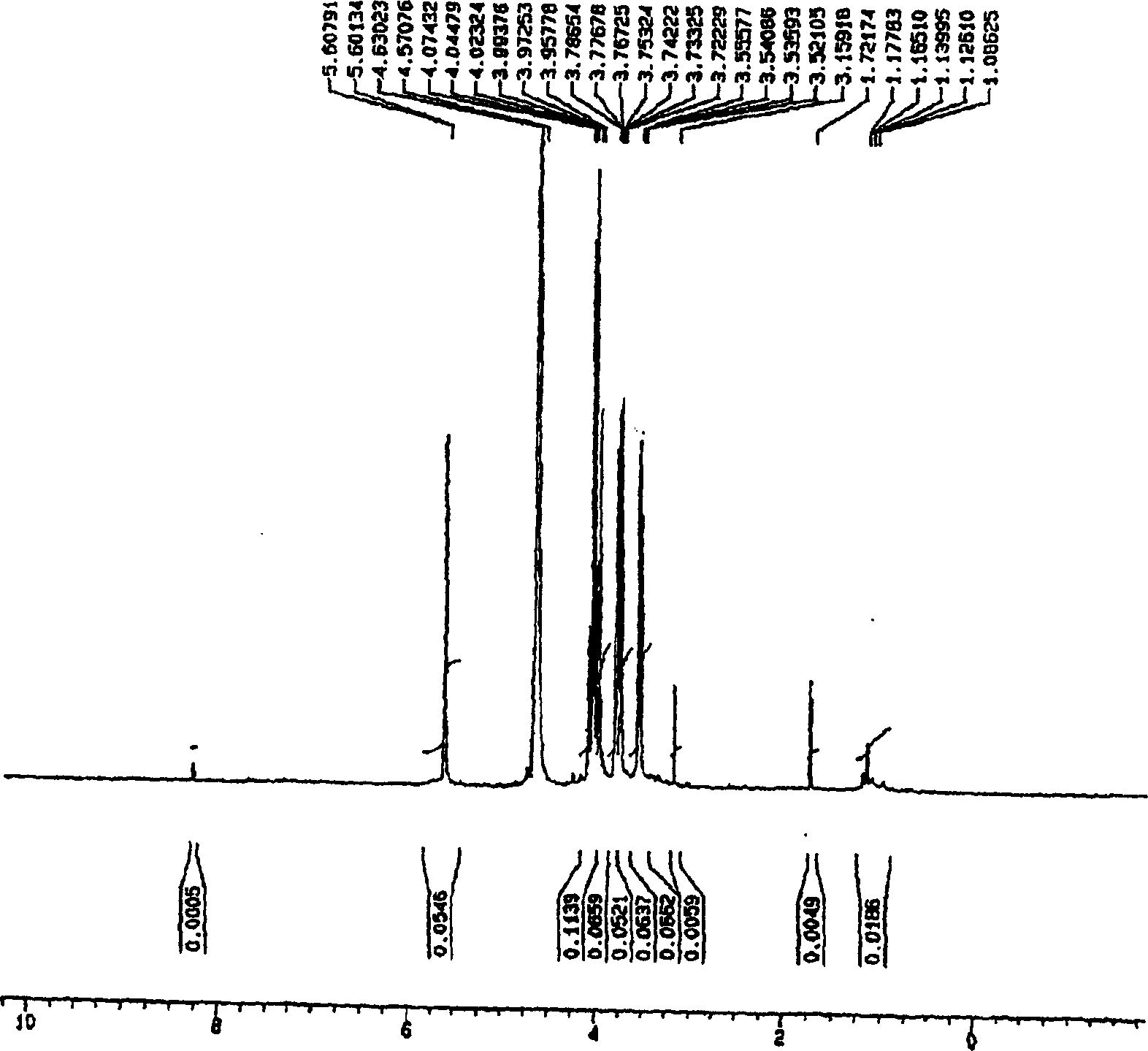
[0031] Embodiment 2: the method that uses TFA to produce visleytamide
[0032] 1 g of pure acarbose derivatives (monosaccharides and trisaccharides) was put into 10% TFA solution to make the final concentration 5%. At a reaction temperature of 100° C., the system was allowed to react for 12 hours or more, followed by removal of TFA and water. Purification was then carried out by using an ion exchange resin to obtain 0.45 g and 0.31 g of vistinide, respectively.
Embodiment 3
[0033] Example 3: Method for the production of Villetamide with TFA by using an autoclave
[0034] Put 10 g of pure acarbose into 10% TFA solution to make the final concentration 5%. While raising the pressure using an autoclave at a reaction temperature of 121° C., the system was allowed to react for 30 minutes to 1 hour, followed by removal of TFA and water. Purification was then performed by using an ion exchange resin to obtain 2.1 g of villetamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com