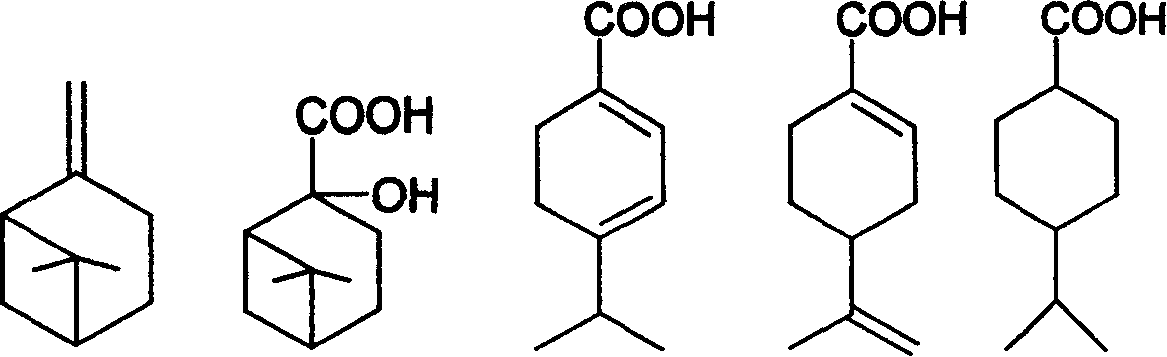
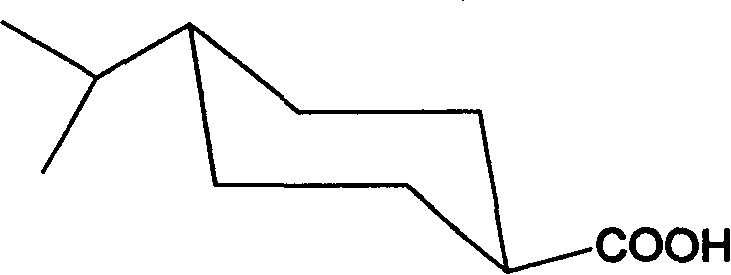
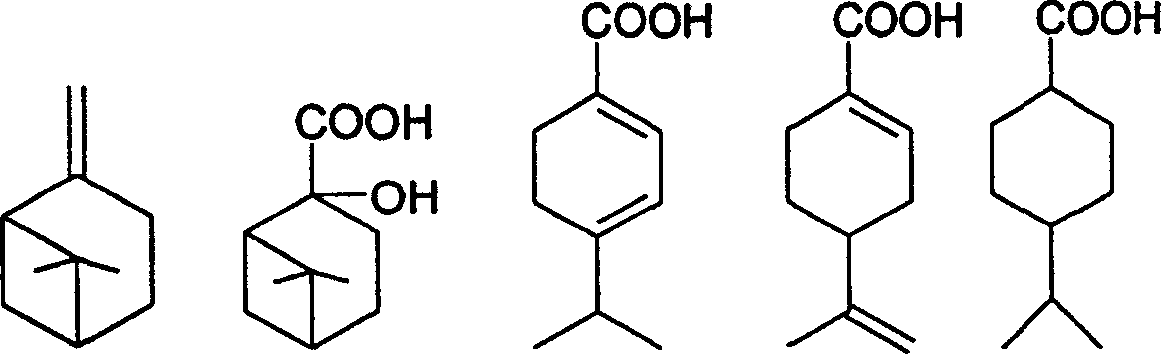
Preparation of trans-4-isopropyl cyclonaphthenic acid
A technology of propylcyclohexanecarboxylic acid and propylcyclohexane, applied in the field of preparation of trans-4-isopropylcyclohexanecarboxylic acid, can solve the problem of high price of 4-isopropylcyclohexanecarboxylic acid and catalytic hydrogenation of benzene ring Low activity, low total reaction yield and other problems, to achieve the effect of abundant raw materials, low cost and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 31g of potassium permanganate, 4g of sodium hydroxide and 400g of water into a three-necked flask with an electric stirrer, add 13.6g of β-pinene in four times within 2 hours, and control the reaction temperature at 15-50°C in an ice bath , After the addition, react at room temperature for 5 hours and stop. After filtration, the filtrate was evaporated to remove water to obtain a white solid of nopinate sodium. The white solid was acidified and extracted three times with chloroform, and the chloroform layer was evaporated to remove the solvent to obtain 6.0 g of nopinate.
[0023] Add 10 g of nopinic acid and 200 mL of 20% sulfuric acid aqueous solution to reflux for 2 hours, cool, and filter to obtain 8 g of the product.
[0024] 10g of the product from the previous step, 0.4g of Pd-C and 60g of ethanol, evacuated hydrogen gas, repeated three times, repeated hydrogen gas at room temperature until the hydrogen pressure no longer drops, stop; filter, evaporate ethano...
Embodiment 2
[0027] Add 43g of potassium permanganate, 4g of sodium hydroxide and 600g of water into a three-necked flask with an electric stirrer, add 13.6g of β-pinene in four times within 2 hours, and control the reaction temperature in an ice bath at 15-50°C. After the addition, react at room temperature for 6 hours and stop. After filtration, the filtrate was evaporated to remove water to obtain 6.6 g of sodium norpinate as a white solid.
[0028] Add 10 g of sodium pinoleate and 200 mL of 20% sulfuric acid aqueous solution to reflux for 2 hours, cool and filter to obtain 7.6 g of the product.
[0029] 10g of the product from the previous step, 0.3g of Pd-C, 60g of methanol, evacuate hydrogen, repeat three times, repeat hydrogen at room temperature until the hydrogen pressure no longer drops, stop; filter, distill off methanol under reduced pressure to obtain 4-isopropyl Cyclohexanecarboxylic acid 9.2g.
[0030] Dissolve 17g of 4-isopropylcyclohexanecarboxylic acid in 100g of toluen...
Embodiment 3
[0032] The data of the melting point, hydrogen spectrum and mass spectrum of embodiment 1,2 product are as follows:
[0033] Melting point: 93~95℃
[0034] Hydrogen spectrum: 1 HNMR (CDCl 3 ): δ=0.85(d, 6H), 1.2(m, 3H), 1.5(m, 3H), 1.8(d, 2H), 2.1(d, 2H), 2.3(m, 1H)
[0035] Mass spectrum: MS (EIMS, 70ev), the m / z value of relevant mass point and fragment: 170 (M + , 10), 152 (M-H 2 O, 12), 127 (M-Pro, 30), 109 (47), 81 (100), 67 (13), 41 (22).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com