Treatment of central nervous system disorders with selective estrogen receptor modulators
A central nervous system, patient technology, applied in the medical field, can solve problems such as increasing reproductive tissue cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
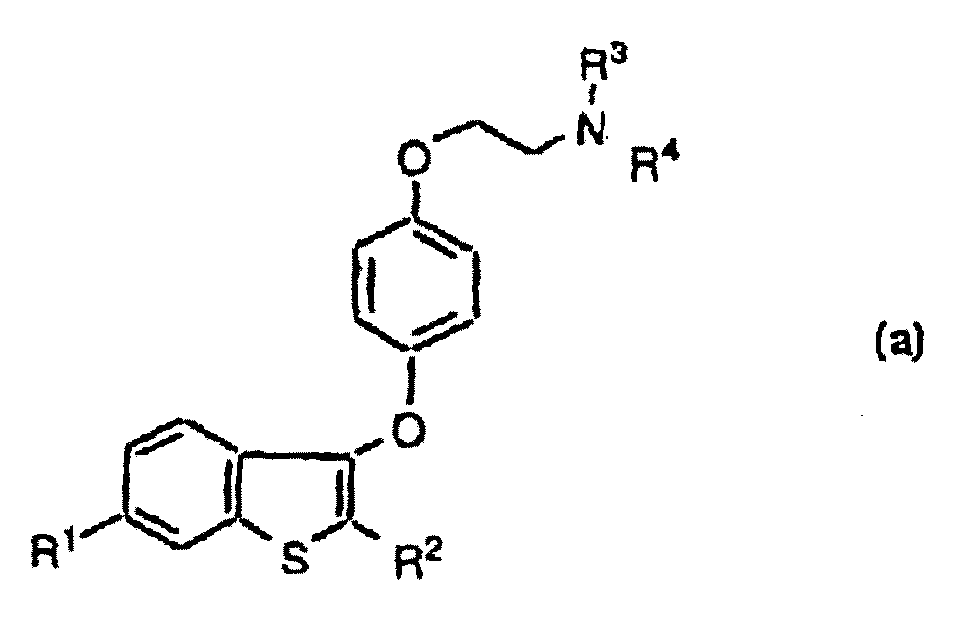
[0062] The preparation of the ester prodrug compound of formula I is by, with formula-OCO-(C 1 -C 6 Alkyl) group or -OSO 2 (C 2 -C 6 Alkyl) groups displace any hydroxyl groups that may be present in the 6- and / or 4'-position by known methods, see, for example, US Pat. No. 4 358 593.
[0063] For example, when -OCO-(C 1 -C 6 Alkyl) is the desired group and a mono- or di-hydroxyl compound of formula I is reacted with reagents such as acid chlorides, bromides, cyanides or azides, or with appropriate anhydrides or mixed anhydrides. The reaction is usually carried out in a basic solvent such as pyridine, lutidine, quinoline or isoquinoline, or in a tertiary amine solvent such as triethylamine, tributylamine, methylpiperidine and the like. This reaction can also be reacted in an inert solvent such as ethyl acetate, dimethylformamide, dimethyl sulfoxide, dioxane, dimethoxyethane, acetonitrile, acetone, methyl ethyl ketone, etc., in such an inert solvent should have added at le...
Embodiment 1
[0114] Example 1 [6-methoxy-3-[4-[2-(1-piperidinyl)ethoxy]-phenoxy]-2-(4-methoxyphenyl)]benzo[ b] Preparation of Thiophene Oxalate Step a: Preparation of [6-methoxy-2-(4-methoxy-phenyl)-3-bromo]benzo[b]thiophene
[0115] At 60°C, to a solution of [6-methoxy-2-(4-methoxyphenyl)]benzo[b]thiophene (27.0g, 100mmol) dissolved in 1.10L chloroform was added dropwise Bromine (15.98 g, 100 mmol) solution in 200 mL chloroform. After the addition was complete, the reaction was cooled to room temperature, and the solvent was removed in vacuo to afford 34.2 g (100%) of [6-methoxy-2-(4-methoxyphenyl)-3-bromo]benzo[b ] Thiophene as a white solid. mp 83-85℃. 1 H NMR (DMSO-d6)d 7.70-7.62 (m, 4H), 7.17 (dd, J=8.6, 2.0Hz, 1H), 7.09 (d, J=8.4Hz, 2H). FD mass spectrum: 349, 350. Theoretical value: C 16 h 13 o 2 SBr: C, 55.03; H, 3.75. Found: C, 54.79; H.3.76. Step b): [6-methoxy-2-(4-methoxyphenyl)-3-(4-benzyl Preparation of oxy)phenoxy]benzo[b]thiophene
...
Embodiment 2
[0119] Example 2 [6-methoxy-3-[4-[2-(1-piperidinyl)ethoxy]-phenoxy]-2-(4-methoxyphenyl)]benzo[ b] Preparation of thiophene hydrochloride
[0120] Treatment of the oxalate salt of Example 1 with aqueous base to generate its free base, followed by reaction with diethyl ether saturated with hydrochloric acid, afforded the title salt. mp 216-220°C. 1 H NMR (DMSO-d6)d 10.20(bs, 1H), 7.64(d, J=8.7Hz, 2H), 7.59(d, J=1.5Hz, 1H), 7.18(d, J=9.0Hz, 1H) , 7.00.(d, J=8.7Hz, 1H), 6.96(dd, J=9.0, 1.5Hz, 1H), 6.92(q, J AB=9.0Hz, 4H), 4.31(m, 2H), 3.83(s, 3H), 3.77(s, 3H), 3.43(m, 4H), 2.97(m, 2H), 1.77(m, 5H), 1.37 (m, 1H). FD mass spectrum: 489. Theoretical value C 29 h 31 NO 4 S.1.0 HCl: C, 66.21; H, 6.13; N, 2.66. Found: C, 66., 46; H, 6.16; N, 2.74.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com