Process for synthesizing 9,9-bisubstituted-2,7-dibromo fluorene or 9,9-bisubstituted fluorene
A technology of dibromofluorene and a synthesis method, which is applied in 9, can solve the problems of low yield, reduced quantum efficiency of luminescent materials, potential safety hazards of raw materials, etc., and achieves the effects of high yield, reduced molecular defects, and little environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
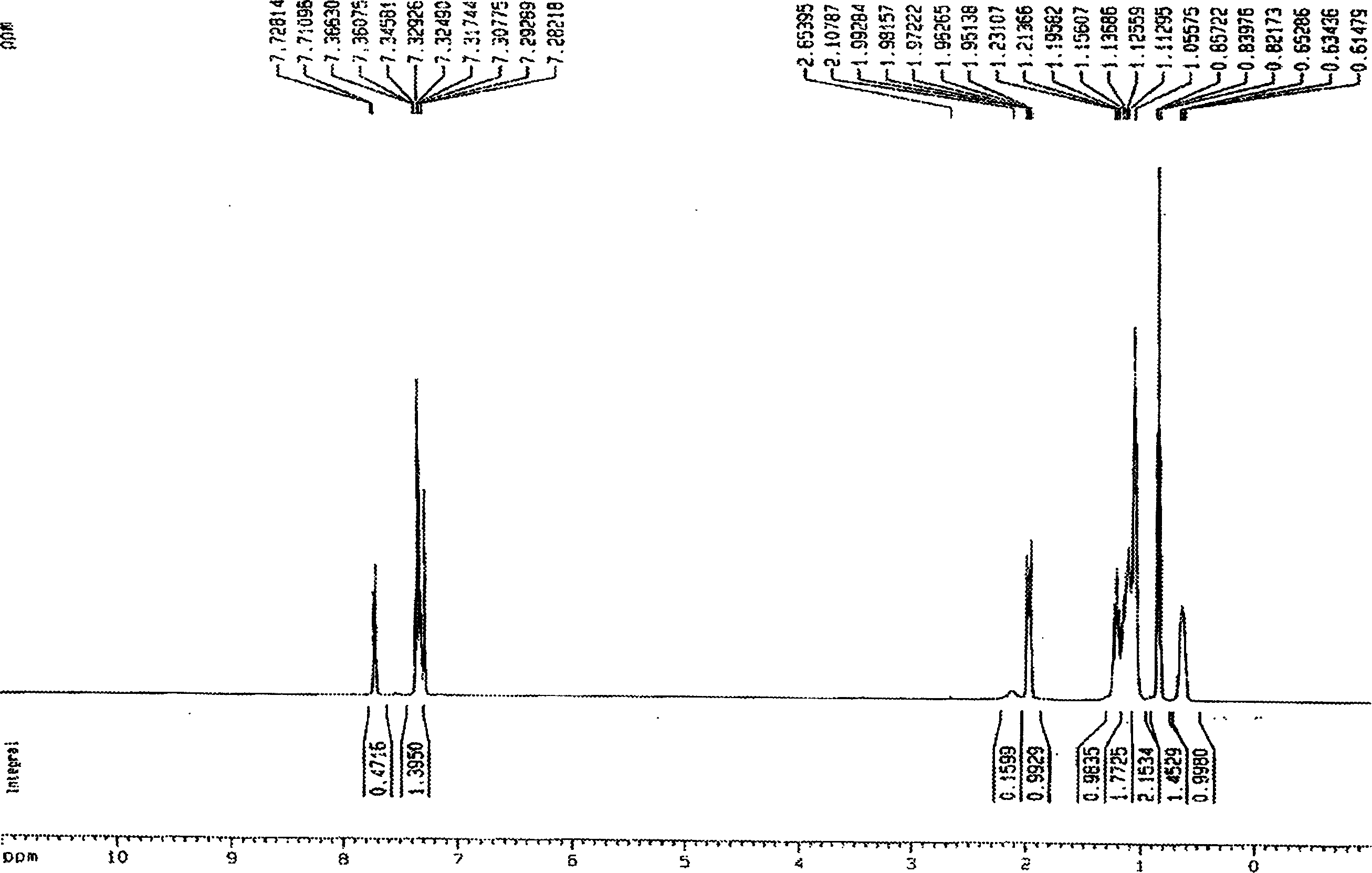
[0028] Embodiment 1 9, the preparation of 9-diethylfluorene
[0029] Under anhydrous, anaerobic and nitrogen protection conditions, ethyl iodide (32ml, 0.40 parts) was dissolved in THF (80ml) to form a homogeneous solution, and then added dropwise to sodium tert-butoxide (48g , 0.50 parts) and fluorene (16.6 g, 0.10 parts) in THF (120 ml) solution. The color of the reaction system changes from blood red-black red-gray white, and the whole process lasts about 2.5 hours.
[0030] After the reaction is completed, the steps of extraction, drying, rotary evaporation, and oil pump to remove the residual solvent are used to obtain a light yellow oily crude product. The crude product was separated and collected by a silica gel column, and finally 21.7 g of a colorless oily liquid 9,9-diethylfluorene was obtained, with a yield of 97.6%.
Embodiment 2
[0031] Embodiment 2 9, the preparation of 9-diethylfluorene
[0032] Under anhydrous, oxygen-free, nitrogen protection conditions, bromoethane (37ml, 0.50 parts) and fluorene (16.6g, 0.10 parts) were dissolved in THF (120ml) to form a homogeneous solution, and then, at a speed of 4ml / min, Add dropwise to a solution of potassium tert-butoxide (67 g, 0.60 parts) in THF (60 ml). The color of the reaction system changes from blood red-black red-gray white, and the whole process lasts about 2.5 hours.
[0033] After the reaction is completed, the steps of extraction, drying, rotary evaporation, and oil pump to remove the residual solvent are used to obtain a light yellow oily crude product. The crude product was separated and collected by a silica gel column, and finally 21.8 g of a colorless oily liquid 9,9-diethylfluorene was obtained, with a yield of 98%.
Embodiment 3
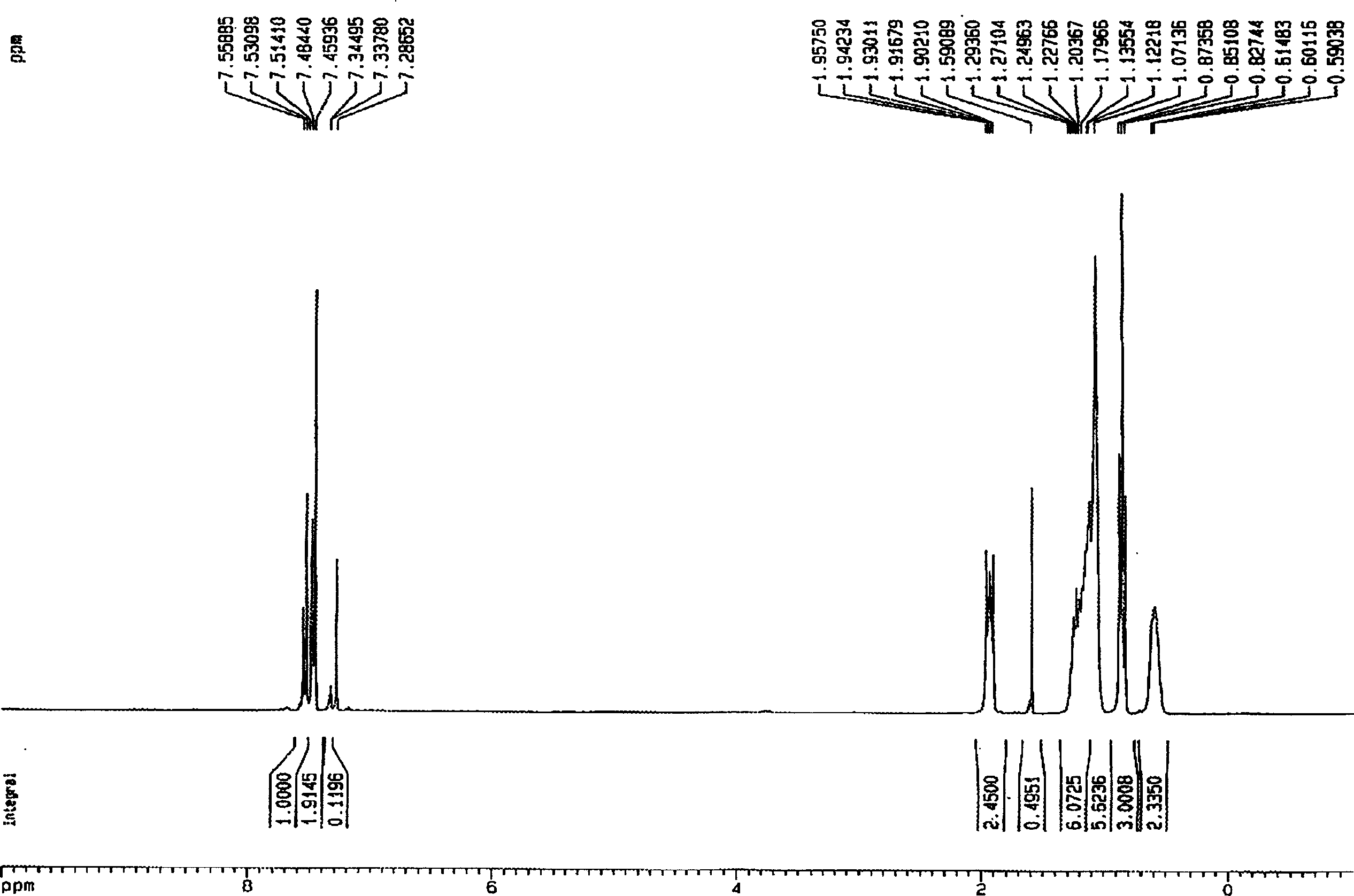
[0034] Example 3 Preparation of 9,9-dibutyl-2,7-dibromofluorene
[0035] Under anhydrous, anaerobic, nitrogen protection conditions, n-bromobutane (27ml, 0.25 parts) was dissolved in THF (100ml) to form a homogeneous solution, then, with a speed of 4ml / min, was added dropwise to sodium tert-butoxide ( 38.5g, 0.40 parts) and 2,7-dibromofluorene (32.4g, 0.10 parts) in THF (150ml) solution. The color of the reaction system changes from blood red-black red-gray white, and the whole process lasts for about 2.5 hours.
[0036] After the reaction is completed, the steps of extraction, drying, rotary evaporation, and oil pump to remove the residual solvent are used to obtain a light yellow oily crude product. The crude product was separated and collected by a silica gel column, and finally 42.2 g of white solid 9,9-dibutyl-2,7-dibromofluorene was obtained with a yield of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com