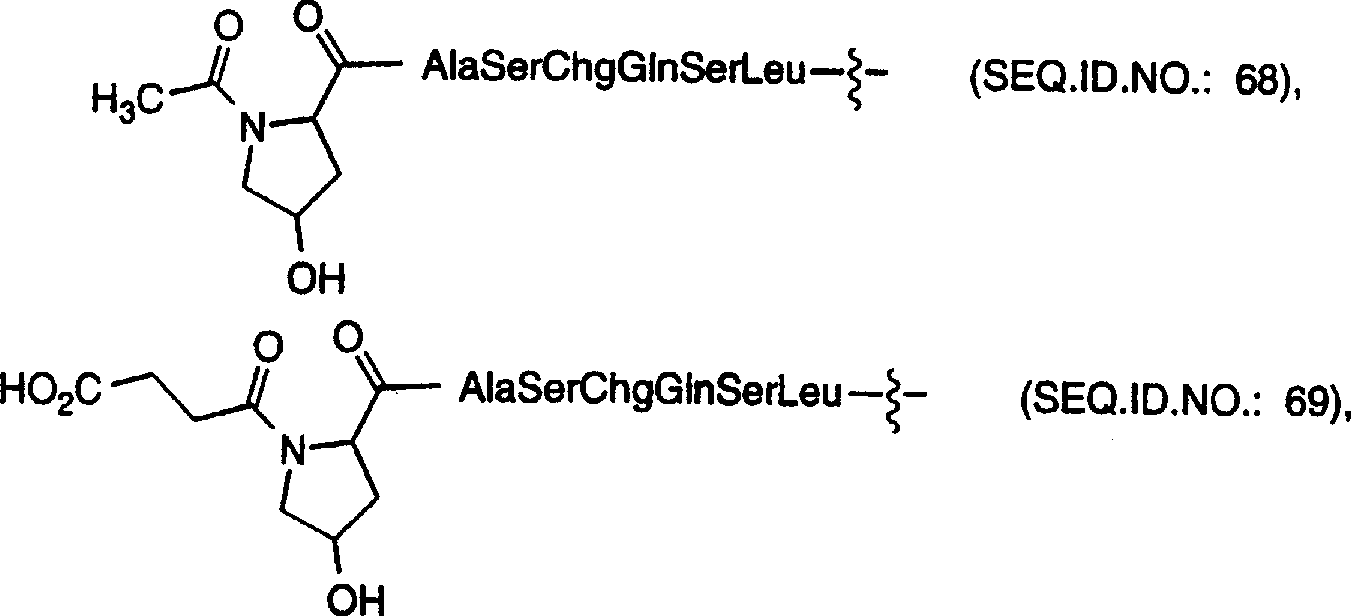Conjugates useful in treatment of prostate cancer
A technology for prostate cancer and conjugates, which can be used in medical preparations and pharmaceutical formulations of non-active ingredients, and can solve problems such as unclear significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0233] Another embodiment of the oligopeptide-cytotoxic agent conjugate of the present invention or a pharmaceutically acceptable salt thereof, which is preferably vinblastine and deacetylvinblastine, described by the following general formula III: in:
[0234] The oligopeptide is an oligopeptide that is selectively recognized by free prostate-specific antigen (PSA) and can be proteolytically cleaved by the enzymatic activity of free prostate-specific antigen, and the oligopeptide includes cyclic amino acids of the following general formula:
[0235] R g and R h independently selected from hydrogen atom, C 1 -C 6 Alkyl, C 1 -C 6 Alkyl-OH,-C 1 -C 6 Alkyl-di-OH, -C 1 -C 6 Alkyl-tri-OH and
[0236] Condition is R d and R e At least one of them is not a hydrogen atom or C 1 -C 6 alkyl, or
[0237] R g and R h Joint Formation - CH 2 CH 2 OCH 2 CH 2 - double radicals;
[0238] R 19 is a hydrogen atom, (C 1 -C 3 Alkyl)-CO or chlorine substituted (C 1 -C...
Embodiment 1
[0274] Preparation of oligopeptides containing a PSA-mediated cleavage site
[0275] In the Applied Biosystems 430A automatic peptide synthesizer, amino acids were introduced by double coupling method, and protected oligopeptides were prepared by solid-phase synthesis. Treatment with liquid hydrofluoric acid achieves the purpose of deprotecting and removing the oligopeptide from the resin support. The oligopeptide was purified by preparative high pressure liquid chromatography on a reverse phase C18 silica gel column eluting with a 0.1% aqueous trifluoroacetic acid / acetonitrile gradient. Oligopeptide identity and homogeneity were confirmed by amino acid composition analysis, high pressure liquid chromatography, and fast atom bombardment mass spectrometry. The oligopeptides prepared by this method are shown in Table 2.
[0276] SEQ.ID.NO.
Peptide / Peptide-DOX Conjugate
Cleavage by PSA
50% substrate required
the time you want
(Min...
Embodiment 2
[0280] Evaluation of oligopeptides recognized by free PSA
[0281] The oligopeptides prepared in Example 1 were individually dissolved in PSA digestion buffer (12mM tris(hydroxymethyl)aminomethane pH8.0, 25mM sodium chloride, 0.5mM calcium chloride) and the solution was mixed in a molar ratio of 100 to 1 added to the PSA. Alternatively, the PSA digestion buffer used was 50 mM tris(hydroxymethyl)-aminomethane pH 7.4, 140 mM sodium chloride. The reaction was stopped after various reaction times by adding trifluoroacetic acid (TFA) to a final concentration of 1% (vol / vol). Alternatively stop the reaction with 10 mM zinc chloride. The quenched reaction was analyzed by HPLC on a reverse phase C18 column eluting with a 0.1% TFA / acetonitrile gradient. The evaluation results are shown in Table 2. Table 2 shows the time (minutes) required for 50% cleavage of the oligopeptides with enzymatically active free PSA. Oligopeptides containing free amine moieties (ie containing hArg, Orn,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com