Method for separating uranium from plutonium in Parex procedure
A process and solution technology, used in the separation of plutonium and uranium in the Praxair process, can solve the problems of complex process and harmful processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
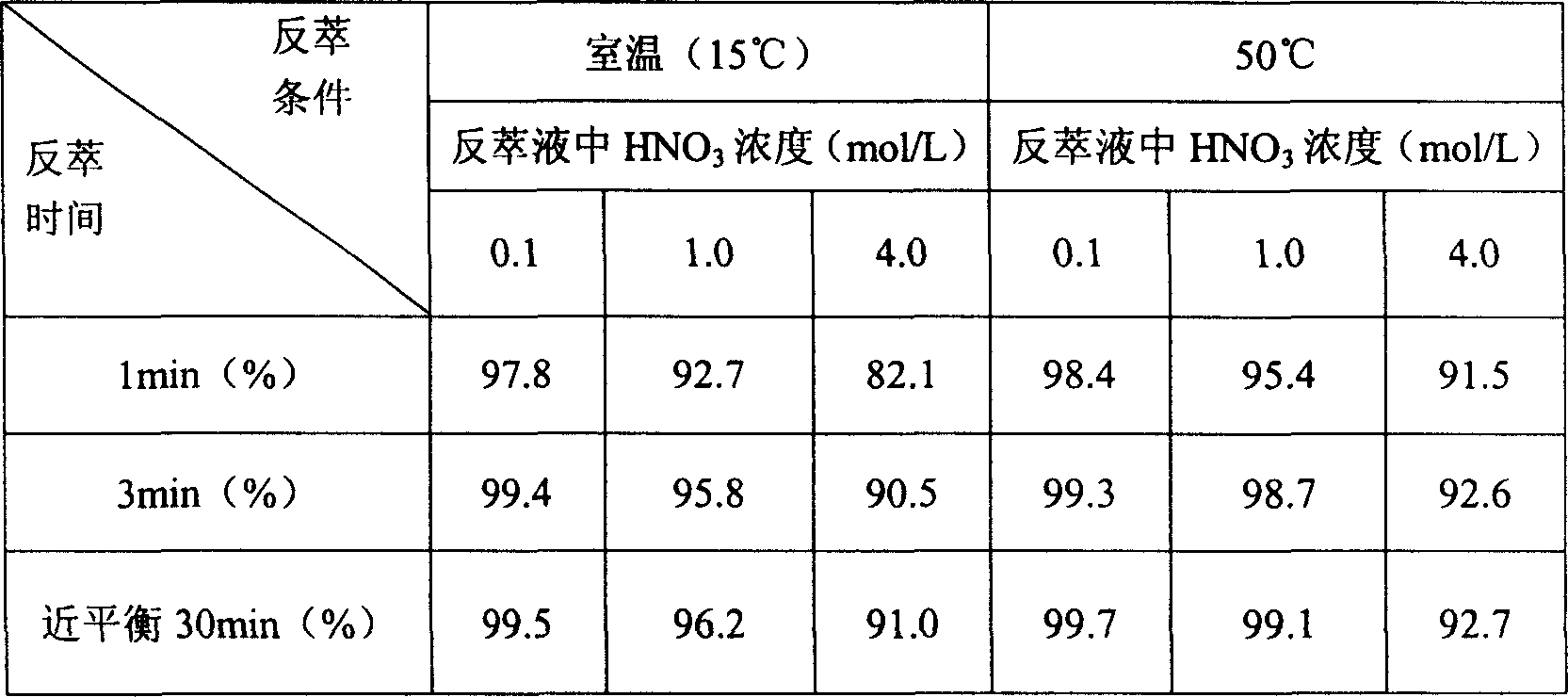
[0006] A method for separating uranium and plutonium in the Purex process, using 0.1-4.0mol / L HNO 3 In the medium, the uranium and plutonium after co-decontamination were co-extracted into 30% tributyl phosphate-kerosene mixture, and the reducing agent hydroxyurea (HU) was added to make the aqueous phase HNO 3 The molar concentration of hydroxyurea in the solution is 10 to 50 times the molar concentration of Pu in the organic phase tributyl phosphate solution, and the aqueous phase HNO 3 The volume ratio of the solution to the organic phase tributyl phosphate solution is 1:1~1:5, fully stirred to reduce Pu(IV) to Pu(III), and Pu(III) is back-extracted to the aqueous phase HNO 3 solution, so as to realize the separation of uranium and plutonium, and the temperature condition of the reaction is 15-50°C.
[0007] Table 1 lists the reduction and stripping effects of different HU and Pu concentration ratios.
[0008] Table 1
[0009] Water phase: C HNO3 =1.0mol / L; organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com