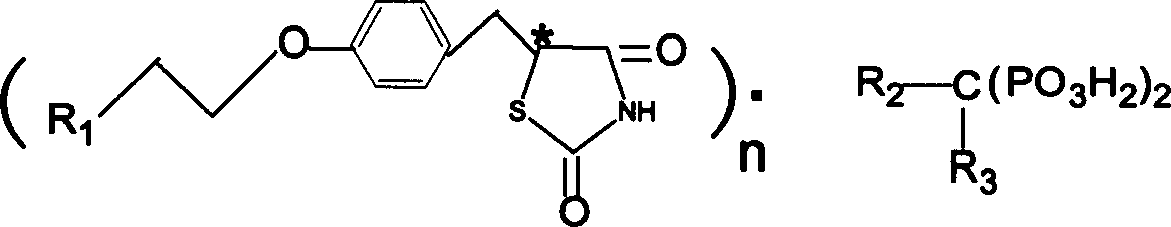
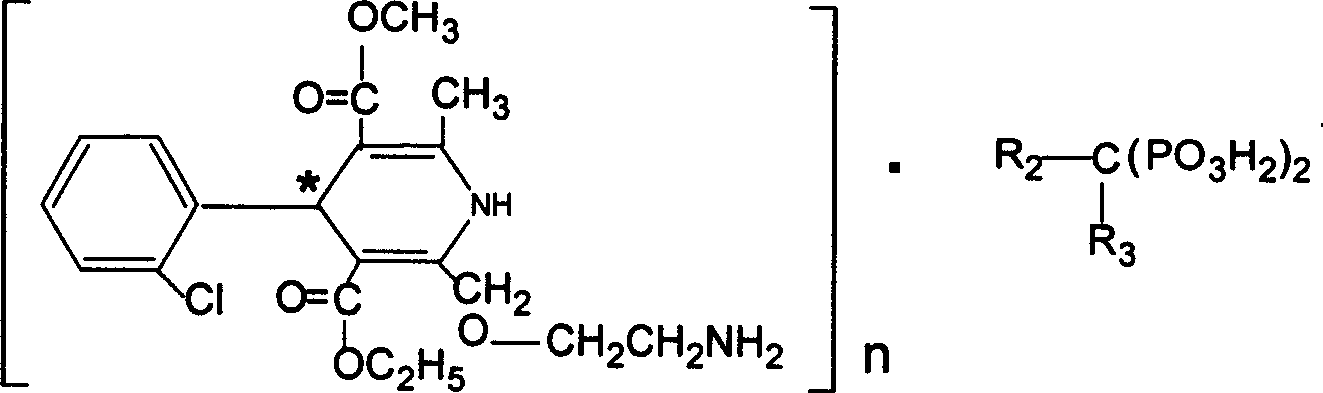Biphosphonate
A technology of bisphosphonic acid and bisphosphonate compounds, which can be used in metabolic diseases, cardiovascular system diseases, organic active ingredients, etc., and can solve problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] etidronate rosiglitazone
[0064] Put 35g of etidronic acid in a 1-liter beaker, add 700ml of distilled water, stir to dissolve into a solution, heat to 70-80°C and add 70g of rosiglitazone in portions while stirring, and add to form a solution, if there is any insoluble matter , removed by filtration. Cool to room temperature, place in the refrigerator overnight, filter, transfer the solid to a beaker, add 120ml of distilled water, stir well, form a paste, filter, drain the mother liquor, wash 3 times with distilled water (3×50ml), dry at 100-104°C for 80- 85 (83-89%), melting point 122-126°C.
[0065] 1 H NMRδ (ppm) (400MHZ, δ 6 -DMSO) 1.43 3.06(δ, 3H), 3.04-3.30(m, 2H), 3.93(t, 2H), 4.14(t, 2H), 4.83-4.86(m, 1H), 6.68(t, 1H), 6.82(d, 1H), 6.86(d, 2H), 7.14(d, 2H), 7.63(t, 1H), 8.05(d, 1H), 12.02(s, 1H)
Embodiment 2
[0067] risedronate amlodipine
[0068] Amlodipine 10.9g is heated and dissolved in hydrochloric acid (0.1008mol M / L, 270ml). If there is any insoluble matter, filter it out, risedronate monosodium 9.6g is dissolved in distilled water 270ml under stirring, and use sodium hydroxide (0.2M / L ) to adjust the pH to 5.4, pour this solution into the amlodipine hydrochloric acid solution at one time, stir at room temperature for 3 hours to form an emulsified suspension, heat and flow for 20 minutes, cool to room temperature, and place in the refrigerator overnight at 100-104°C for drying. 16 g (80.7%) mp 183-5°C.
[0069] 1 H NMRδ (ppm 400MHZ, δ 6 -DMSO) 1.01(t, 3H), 2.31(s, 3H), 3.02(s, 2H), 3.16(t, 2H), 3.50(s, 3H), 3.66(s, 2H), 4.01(m, 2H ), 4.60(dd, 2H), 5.29(s, 1H), 7.10-7.25(m, 4H), 7.35(d, 1H), 7.88(d, 1H), 8.26(d, 1H) 8.56(s, 1H ), 8.74(s, 1H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com