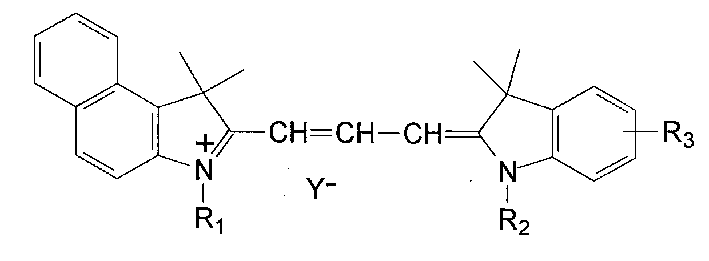
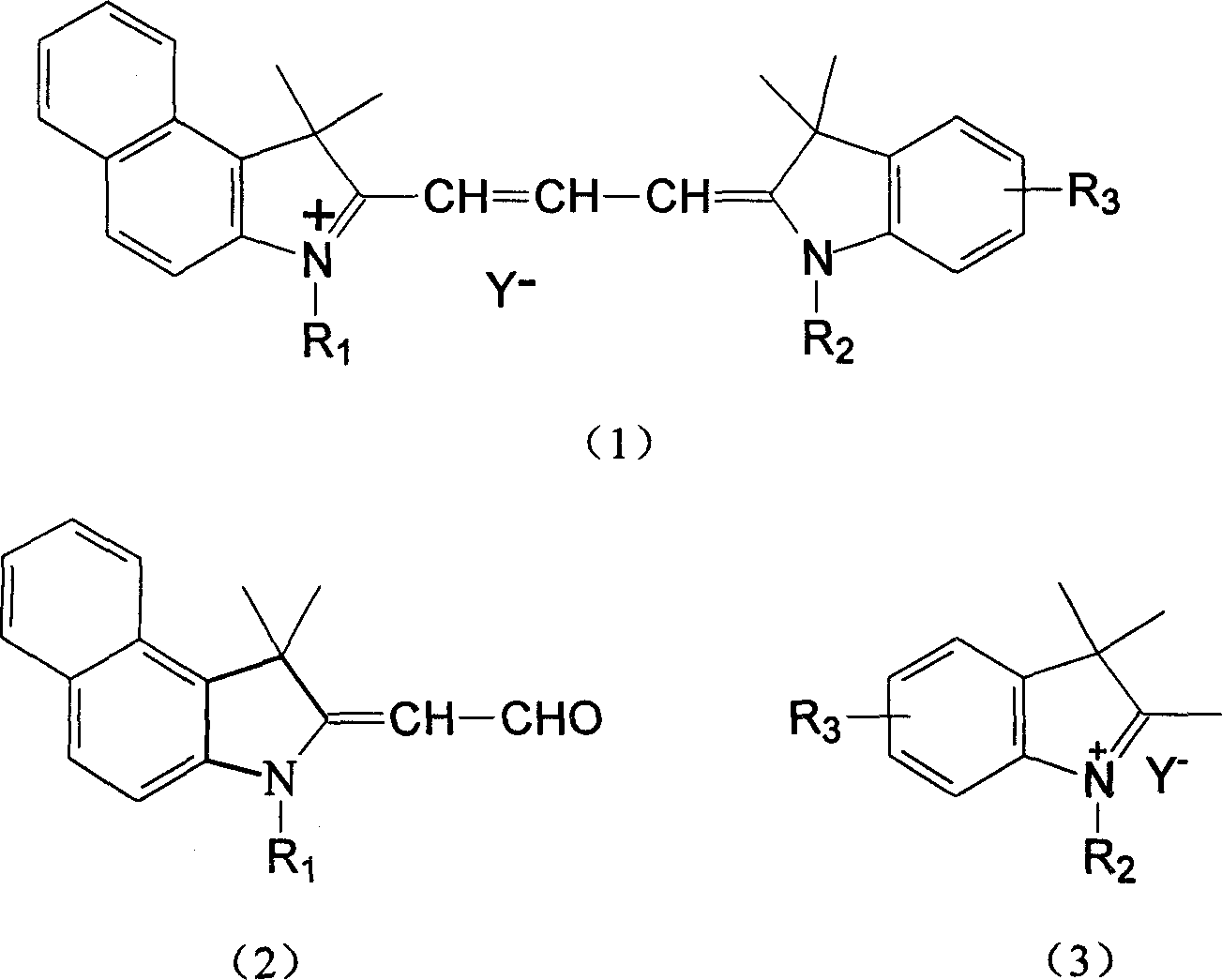
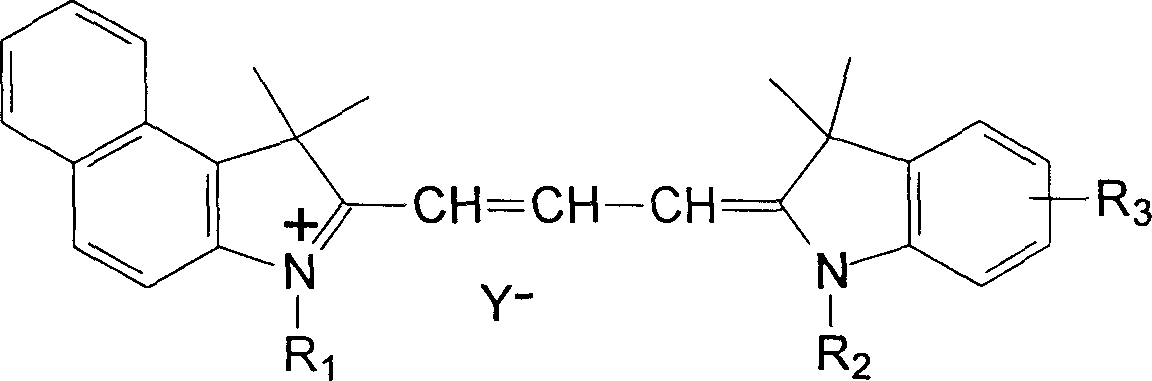Preparation of asymmetric cyanine dyes for DVD-R CD
A technology for asymmetric cyanine dyes, applied in the field of preparation of asymmetric cyanine dyes, can solve the problems of difficult purification and low purity, and achieve the effects of high purity of raw materials, good solubility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of Dye CY-3-1:
[0041]
[0042] Put 15g 1,1-dimethyl-3-butyl-2-formylmethylene-1H-benzo[e]indole, 17-6g 2,3,3-trimethyl-1-butyl Put -3H-indole iodide salt and 150ml acetic anhydride into a three-neck flask, start stirring, and heat to 105-110°C for 1 hour to react. Then, the reaction solution was slowly poured into 120ml of water under stirring, and the stirring was continued until a loose red solid was precipitated, filtered, washed with water for 2 to 3 times, and the filtered solid was dried. The solid was dissolved in methanol under heating and reflux, 18 g of sodium perchlorate crystals were added, the reflux was continued for 1 hour, and the product was naturally cooled to room temperature, 4.9 g of the product was crystallized, and the yield was 87%.
[0043] m.p.199~201℃.
[0044] 1 H-NMR (d 6 -DMSO) (ppm):
[0045] 0.97(t, 6H), 1.46(m, 4H), 1.75(m, 10H), 1.99(s, 6H), 4.14(t, 2H), 4.28(t, 2H), 6.55(t, 2H), 7.31 (m, 1H), 7.46(d, 1H), 7.55(t, 2...
Embodiment 2
[0047] Synthesis of Dye CY-3-2:
[0048]
[0049] Replace 2,3,3-trimethyl-1-butyl-3H-indole iodide in Example 1 with 1,2,3,3-tetramethyl-3H-indole iodide, other and implementation Example 1 is the same, and the yield is 89%.
[0050] m.p.142-144°C.
[0051] 1 H-NMR (d 6 -DMSO) (ppm):
[0052] 0.94(t, 3H), 1.45(m, 2H), 1.73(m, 8H), 1.96(s, 6H), 3.65(s, 3H), 4.24(t, 2H), 6.50(m, 2H), 7.29 (m, 1H), 7.44(d, 2H), 7.52(t, 1H), 7.66(t, 2H), 7.78(d, 1H), 8.08(m, 2H), 8.30(d, 1H), 8.46( t, 1H).
Embodiment 3
[0054] Synthesis of Dye CY-3-3:
[0055]
[0056] Put 15.5g 1,1-dimethyl-3-butyl-2-formylmethylene-1H-benzo[e]indole, 20g 5-chloro-2,3,3-trimethyl-1 -Butyl-3H-indole iodide salt and 150ml of acetic anhydride were put into a three-neck flask, started stirring, and heated to 105-110°C for 1 hour to react. Then slowly pour the reaction solution into a solution formed by dissolving 24g of potassium hexafluorophosphate in 120ml of water under stirring, and continue stirring until a loose red solid precipitates, filter, wash with water 2 to 3 times, and dry. The solid was dissolved in methanol under reflux, and naturally cooled to room temperature, 30.5 g of the product was crystallized, with a yield of 86%.
[0057] m.p.220~222℃.
[0058] 1 H-NMR (d 6 -DMSO) (ppm):
[0059] 1.01(m, 6H), 1.63(m, 4H), 1.74(s, 6H), 1.85(m, 2H), 1.92(m, 2H), 2.01(s, 6H), 4.24(t, 2H), 4.45 (t, 2H), 7.01(d, 1H), 7.32(s, 1H), 7.35(d, 1H), 7.42~7.44(m, 2H), 7.50~7.57(m, 2H), 7.63(t, 1H) ), 7.97 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| reflectance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com