Triazine compound as well as preparation method and application thereof
A compound and triazine technology, applied in the field of preparation of triazine compounds, can solve the problems of single structure, the lack of non-covalent high-efficiency 3CLpro small molecule inhibitors, etc., and achieve small toxic side effects, good inhibitory activity, good effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Synthesis of compound IV-1
[0070]
[0071] Step 1: Synthesis of Compound 2
[0072] To a solution of compound 2 (100.0 mg, 0.47 mmol) in N,N-dimethylformamide (10 mL), potassium hydroxide (105.5 mg, 1.88 mmol) and iodine (239 mg, 0.94 mmol) were added, and the reaction was carried out at room temperature. After 3 hours, TLC monitored the completion of the reaction. Saturated sodium sulfite solution was added to quench the reaction. The aqueous phase was extracted with ethyl acetate (10 mL*2), washed with water (20 mL*2), and washed with saturated salt (20 mL) with anhydrous sodium sulfate. Dry and concentrate by column chromatography to obtain compound 3 (134.8 mg, 85%). 1 HNMR (300 MHz, CDCl 3 ) δ 8.79 (s, 1H), 7.70 (s, 1H), 3.90 (s, 3H).
[0073] Step 2: Synthesis of Compound 3
[0074] To a solution of compound 3 (120.0 mg, 0.36 mmol) in deuterated acetic acid (8 mL), sodium acetate (97.9 mg, 0.72 mmol) was added, the dropwise addition was complete...
Embodiment 2
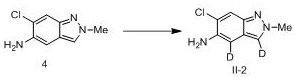
[0077] Example 2 Synthesis of compound IV-2
[0078]
[0079] To compound 4 (30 mg, 0.17 mmol) was added deuterated acetic acid (2 mL), refluxed at 120 °C for 25 hours, TLC monitored the completion of the reaction, and concentrated under reduced pressure to obtain compound IV-2 (28 mg, 92%). 1 H NMR (300 MHz, CDCl 3 ) δ 7.44(s, 1H), 5.11 (d, J = 7.1 Hz, 1H), 4.95 (d, J = 7.1 Hz, 1H), 3.85 (s, 3H).
Embodiment 3
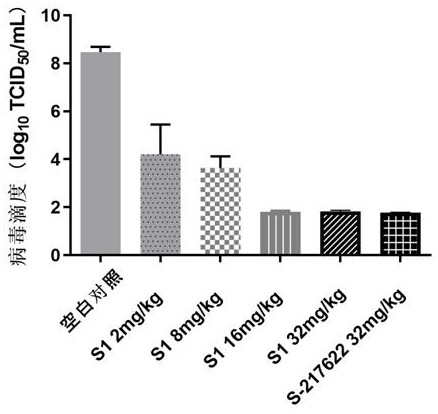
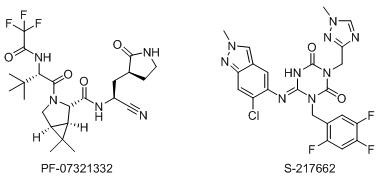
[0080] Example 3: Synthesis of Compound S1
[0081]
[0082] Step 1: Synthesis of Compound 6
[0083] Compound 5 (18 g, 78.8 mmol) was dissolved in acetonitrile (240 mL), to the above solution was added compound 11 (26 g, 118.8 mmol) and K 2 CO 3 (16.4 g, 118.8 mol), the reaction solution was heated to reflux for 3 h. The reaction solution was cooled to room temperature, filtered with suction, the filtrate was concentrated, and separated and purified by column chromatography (PE:EA=30:1) to obtain compound 6 (23.5 g, 80%). 1 H NMR (300 MHz, CDCl 3 ) δ 1.33 (3H, t, J = 7.4 Hz), 1.65 (9H, s), 3.15 (2H, q, J =7.4 Hz), 5.03 (2H, s), 6.91−7.01 (2H, m).
[0084] Step 2: Synthesis of Compound 7
[0085] Compound 6 (20 g, 51.9 mmol) was dissolved in TFA (39 mL), the reaction was stirred at room temperature for 6 h, the stirring was stopped, the TFA was evaporated under reduced pressure, slurried with ether, filtered with suction, the filter cake was collected, and dried in vac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com