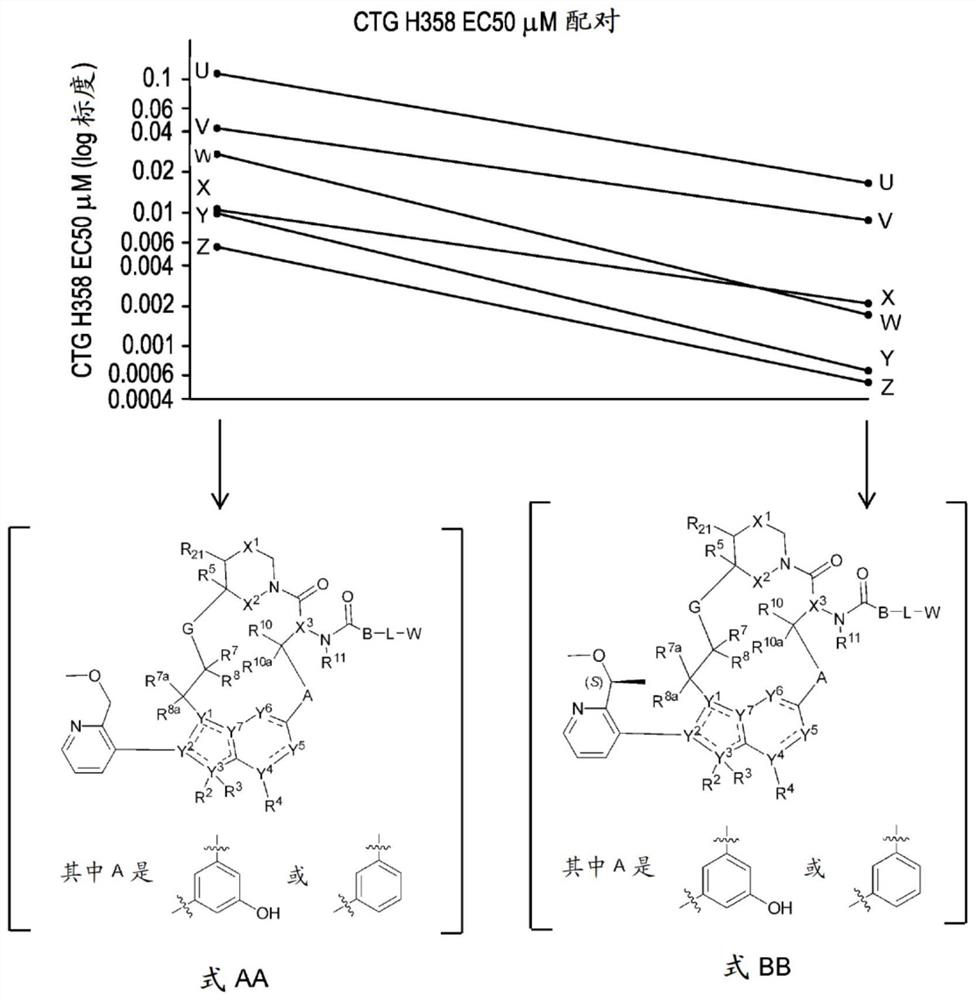
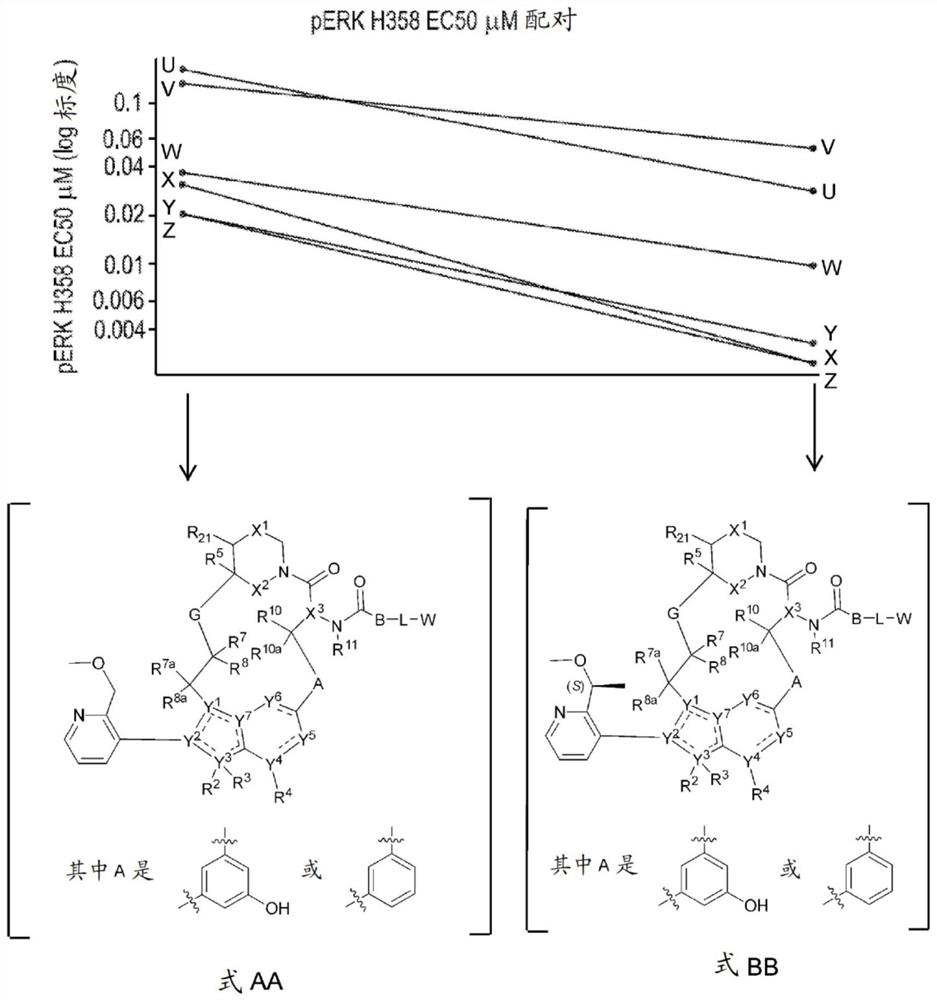
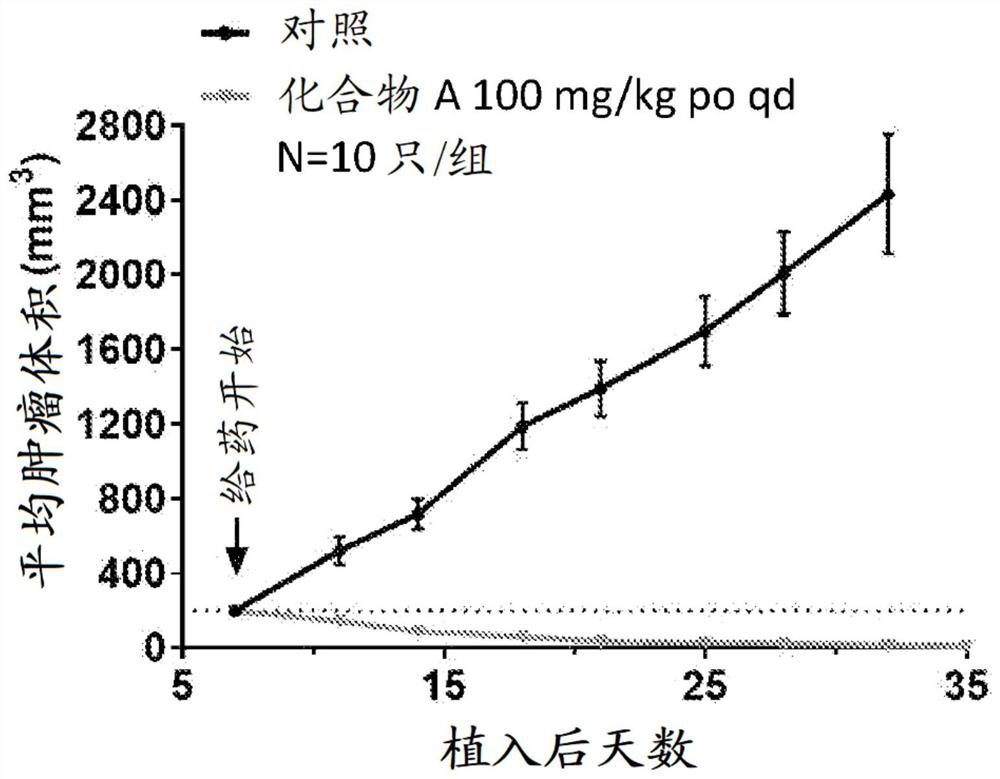
RAS inhibitors
A pharmaceutical, optional technology, applied in the field of RAS inhibitors, which can solve problems such as unapproved drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[1073] [1] A compound or a pharmaceutically acceptable salt thereof having the structure of formula I:
[1074]
[1075] where dashed lines represent zero, one, two, three or four non-adjacent double bonds;
[1076] A is -N(H or CH 3 )C(O)-(CH 2 )-, where the amino nitrogen is bound to -CH(R 10 )- carbon atom; optionally substituted 3- to 6-membered cycloalkylene; optionally substituted 3- to 6-membered heterocycloalkylene; optionally substituted 6-membered arylene; or optionally substituted 5- to 10-membered heteroarylene;
[1077] B does not exist and is -CH(R 9 )-, >C=CR 9 R 9' or >CR 9 R 9' , where the carbon is bound to -N(R 11 ) carbonyl carbon of C(O)-; optionally substituted 3- to 6-membered cycloalkylene; optionally substituted 3- to 6-membered heterocycloalkylene; optionally substituted 6-membered aryl; or 5- to 6-membered heteroarylene;
[1078] G is optionally substituted C 1 -C 4 Alkylene; optionally substituted C 1 -C 4 Alkenylene; optionally su...
Embodiment
[1488] The present disclosure will be further illustrated by the following examples and synthetic examples, which should not be construed to limit the scope or spirit of the present disclosure to the specific procedures described herein. It should be understood that the examples are provided to illustrate certain embodiments and are not intended to thereby limit the scope of the present disclosure. It should also be understood that various other embodiments, modifications and equivalents thereof, which may occur to those skilled in the art, may also be resorted to without departing from the spirit of the present disclosure or the scope of the appended claims.
[1489] chemical synthesis
[1490] Definitions used in the following examples and elsewhere herein are as follows:
[1491] CH 2 Cl 2 , DCM methylene chloride, dichloromethane
[1492] CH 3 CN, MeCN Acetonitrile
[1494] DIPEA Diisopropylethylamine
[1495] DMF N,N-Dimethylformamide...
Embodiment A75
[1590] Example A75. Synthesis of (2S)-N-[(8S,14S,20M)-22-ethyl-4-hydroxy-21-{2-[(1S)-1-methoxyethyl]pyridine-3 -yl}-18,18-dimethyl-9,15-dioxo-16-oxa-10,22,28-triazapentacyclo[18.5.2.1 2,6 .1 10,14 .0 23,27 ] Nonacosa-1(26),2,4,6(29),20,23(27),24-heptaen-8-yl]-3-methyl-2-{N-methyl- Two atropisomers of 1-[(3S)-1-(prop-2-enoyl)pyrrolidin-3-yl]carboxamido}butanamide.
[1591]
[1592] Step 1. At 0°C, to ((6 3 S,4S)-1 1 -Ethyl-1 2 -(2-((S)-1-methoxyethyl)pyridin-3-yl)-10,10-dimethyl-5,7-dioxo-2 5 -((triisopropylsilyl)oxy)-6 1 ,6 2 ,6 3 ,6 4 ,6 5 ,6 6 - Hexahydro-1 1 H-8-oxa-1(5,3)-indoleza-6(1,3)-pyridazine-2(1,3)-benzcycloundecan-4-yl)carbamic acid tert. To a stirred mixture of butyl ester (18.0 g, 20.1 mmol) in THF (180 mL) was added 1 M TBAF in THF (24.1 mL, 24.1 mmol). The mixture was stirred at 0 °C for 1 hour, then diluted with brine (1.5 L) and extracted with EtOAc (3 x 1 L). The combined organic layers were washed with brine (2 x 500 mL), washed with anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com