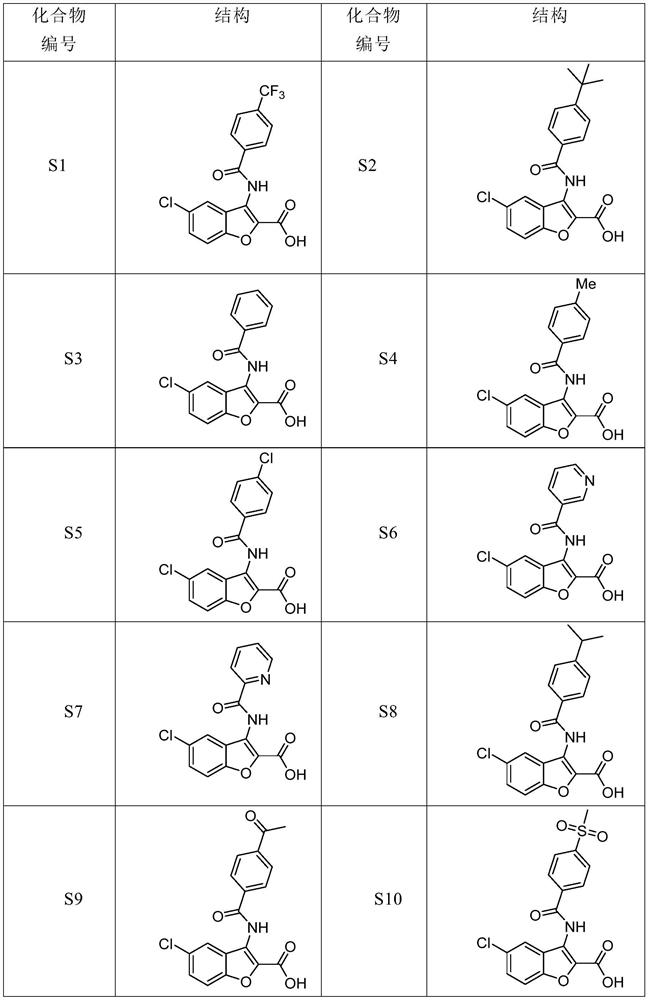
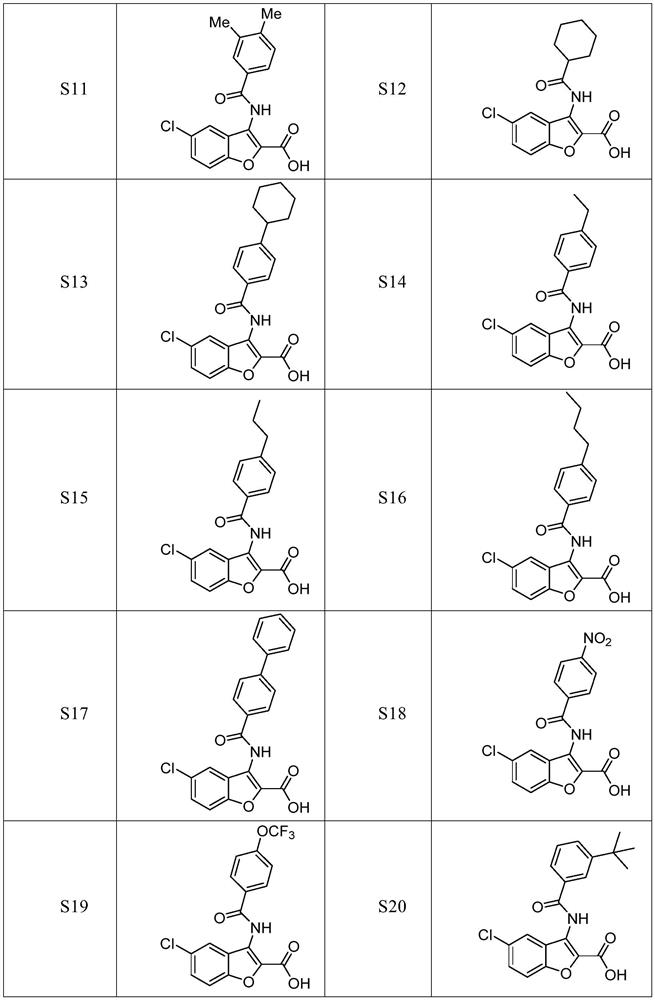
Benzofuran derivative and medical application thereof
A technology of benzofuran and derivatives, which is applied in the field of benzofuran derivatives and their medical applications, can solve the problem of single compound structure, and achieve the effect of highlighting the substantial characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0224] Inhibitory effect of compounds on cGAMP-stimulated STING pathway in THP1-Dual cells by luciferase assay
[0225] 1) 4*10 THP1-Dual cells in logarithmic growth phase 4 The samples / well were seeded in 96-well plates, and different concentrations of compounds were added, and the DMSO group was used as a blank control.
[0226] 2) The cells were transfected with 10ug / mL cGAMP and lipofectamine 2000 (Invitrogen) complex and cultured for 24h.
[0227] The preparation method of the transfection complex: 1ug cGAMP was added to 10uL Opit-MEM, 0.5uL Lipofectamine2000 was added to 10uL Opti-MEM, mixed and left to stand for 15 minutes, and 20uL of the complex was added to the 96-well plate.
[0228] 3) Using QUANTI-Luc TM Reagents to detect luciferase activity. Take 10uL of cell culture supernatant and add it to a 96-well opaque white plate, then add 50uL of QUANTI-Luc TM Reagents were read with a multi-plate reader (Thermo).
[0229] Calculation method of relative luciferase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com