Preparation method and application of aza fused ring g-C3N4 composite material
A composite material, g-c3n4 technology, applied in the field of preparation of aza-fused ring g-C3N4 composite materials, can solve the problems of high electron and hole recombination rate, low surface area, band bending, etc., and achieves remarkable progress and degradation. The effect of high efficiency and high practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
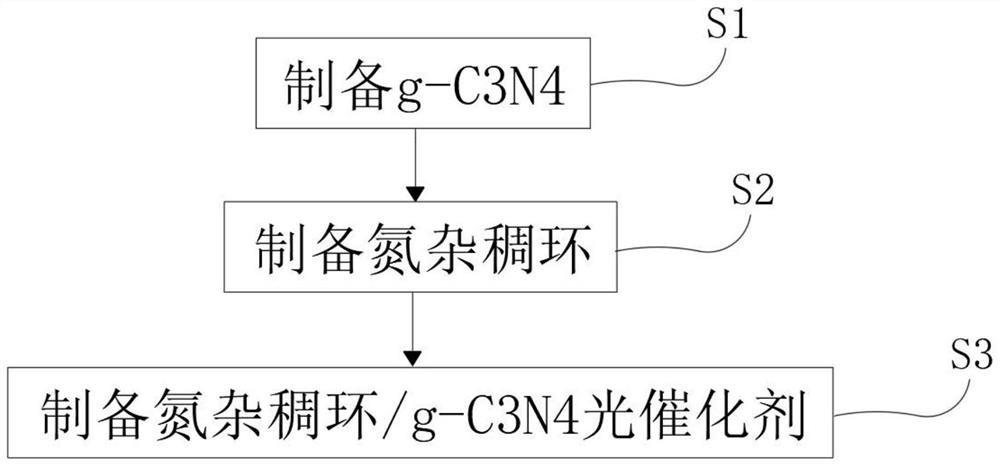
[0020] see figure 1 , a preparation method of aza-fused ring g-C3N4 composite material, the preparation method of described aza-fused ring g-C3N4 composite material comprises the following steps:
[0021] S1, preparation of g-C 3 N 4 , prepare g-C by direct pyrolysis of 20g melamine in a tube furnace under nitrogen protection atmosphere 3 N 4 , keep it for future use;
[0022] S2, prepare aza-fused ring, add 1 g of cyclohexanone octahydrate and 1.3 g of 1,2,4,5-phenylenetetramine tetrahydrochloride into a round-bottomed flask under nitrogen atmosphere, and then add 0.5 mL of H 2 SO 4 Mixed solution with 100 mL of deoxygenated N-methylpyrrolidone, then the round-bottomed flask was placed in an ice bath and stirred for 2 hours, then transferred to an oil bath and heated to 180°C, followed by a continuous reaction for 8 hours and the device was cooled to room temperature, Water was added to quench the reaction, and then suction filtered to obtain a black solid product, and...
Embodiment 2
[0029] see figure 1 , a preparation method of aza-fused ring g-C3N4 composite material, the preparation method of described aza-fused ring g-C3N4 composite material comprises the following steps:
[0030] S1, preparation of g-C 3 N 4 , prepare g-C by direct pyrolysis of 20g melamine in a tube furnace under nitrogen protection atmosphere 3 N 4 , keep it for future use;
[0031] S2, prepare aza-fused ring, add 1.5g of cyclohexanone octahydrate and 2g of 1,2,4,5-phenylenetetramine tetrahydrochloride into a round-bottomed flask under nitrogen atmosphere, and then add 2mL of H 2 SO 4 Mixed solution with 150 mL of deoxygenated N-methylpyrrolidone, then the round-bottomed flask was placed in an ice bath and stirred for 2 hours, then transferred to an oil bath and heated to 180°C. After continuous reaction for 8 hours, the device was cooled to room temperature. Water was added to quench the reaction, and then suction filtered to obtain a black solid product, and then methanol an...
Embodiment 3
[0038] see figure 1 , a preparation method of aza-fused ring g-C3N4 composite material, the preparation method of described aza-fused ring g-C3N4 composite material comprises the following steps:
[0039] S1, preparation of g-C 3 N 4 , prepare g-C by direct pyrolysis of 20g melamine in a tube furnace under nitrogen protection atmosphere 3 N 4 , keep it for future use;
[0040] S2, prepare aza-fused ring, add 2 g of cyclohexanone octahydrate and 3.3 g of 1,2,4,5-benzenetetramine tetrahydrochloride into a round-bottomed flask under nitrogen atmosphere, and then add 5 mL of H 2 SO 4 Mixed solution with 100 mL of deoxygenated N-methylpyrrolidone, then the round-bottomed flask was placed in an ice bath and stirred for 2 hours, then transferred to an oil bath and heated to 180°C, followed by a continuous reaction for 8 hours and the device was cooled to room temperature, Water was added to quench the reaction, and then suction filtered to obtain a black solid product, and then m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com