Pharmaceutical composition for treating diabetic neuropathy and preparation method thereof
A technology of composition and medicine, which is applied in the fields of pharmaceutical composition for treating diabetic neuropathy and its preparation, epalrestat tablet and its preparation, and can solve the problem of the average dissolution rate of epalrestat tablets and the difference in dissolution rate between tablets. Large and other problems, to achieve the effect of improving bioavailability, increasing average dissolution rate, accelerating release and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prescription: epalrestat 45.45%, mannitol 47%-48%, low-substituted hypromellose 4.0%, hypromellose 1-3%, magnesium stearate 1.0%, appropriate amount of water (to prepare adhesive )
[0036] preparation:
[0037] 1) Micro-differentiate the prescription amount of epalrestat and pulverize to different particle sizes in the following table;
[0038] 2) adding water to the recipe quantity of hypromellose, the preparation hypromellose concentration is 3%;
[0039] 3) adding the micronized epalrestat, mannitol and low-substituted hydroxypropyl cellulose of the recipe quantity into a wet mixing granulator in order for mixing, adding step 2) binder, and granulating;
[0040] 4) in step 3) granule, add recipe quantity lubricant, mix well, press tablet;
[0041] 5) Add the coating material of the recipe quantity, coat, and get the product.
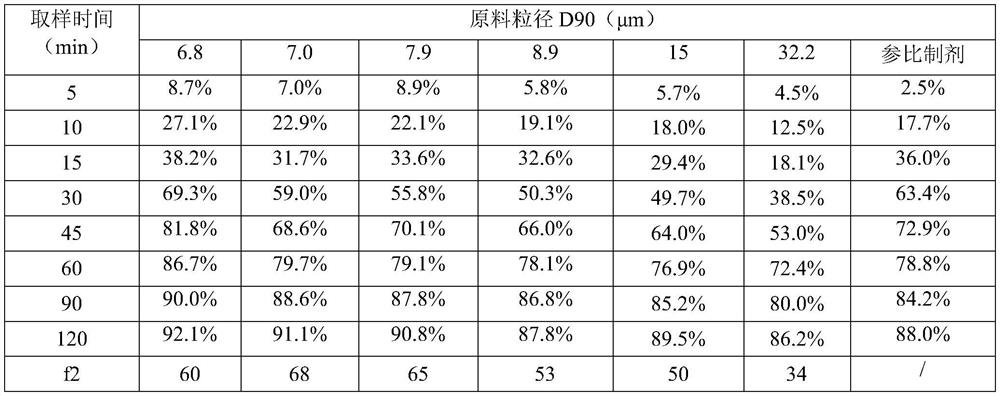
[0042] The dissolution curves of formulations with different particle sizes of raw materials in 1.5% SDS-pH4.5 medium were determined. Th...
Embodiment 2
[0049] Prescription: epalrestat 45.45%, mannitol 46.05%, low-substituted hypromellose 4.00%, hypromellose 3.50%, magnesium stearate 1.00%, appropriate amount of water (to prepare binder)
[0050] Preparation: Same as Example 1.
[0051]Determination of the dissolution curves of formulations with different particle sizes of raw materials in 1.5% SDS-pH4.5 medium:
[0052]
[0053]
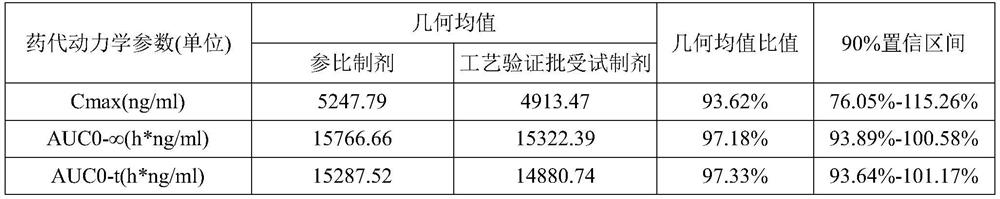
[0054] The results show that when the particle size of the raw material is D90≤6μm, the cumulative dissolution rate at each time point is higher than that of the reference preparation or similar to the reference preparation, and f2>70. Equivalence study, the test results are shown in the table:
[0055]
[0056] The results show that when the particle size D90 of the raw material is less than or equal to 6 μm, the geometric mean value and confidence interval of the prepared preparation are both improved.
Embodiment 3-5
[0058]
[0059] preparation:
[0060] 1) Differentiate the prescription amount of epalrestat to a particle size of D90≤6μm;
[0061] 2) water is added to recipe quantity hypromellose, and is mixed with the aqueous solution of concentration (w / w) 5%;
[0062] 3) take by weighing epalrestat (micronized), mannitol, and the adhesive prepared in step 2) according to the prescribed dosage and transfer it to the fluidized bed for granulation;
[0063] 4) In step 3) the granules are mixed with low-substituted hydroxypropyl cellulose in the recipe quantity, then a lubricant in the recipe quantity is added, mixed evenly, and pressed into tablets.
[0064] Dissolution profiles of different examples in 1.5% SDS-pH 4.5 medium:
[0065]
[0066]
[0067] It can be seen from Examples 3-5 that although the dissolution curves of each formulation in 1.5% SDS-pH4.5 medium are similar to those of the reference formulation, except for 60 and 90 min, the cumulative dissolution at other t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com