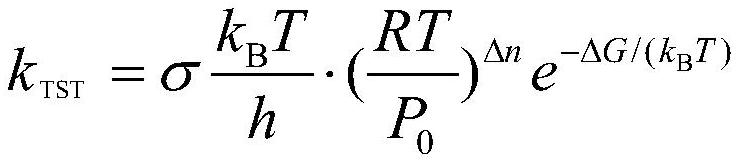Quantum chemistry method capable of calculating gas-phase hydroxyl radical reaction rate constants of various perfluoroalkyl/polyfluoroalkyl compounds
A reaction rate constant, polyfluoroalkyl technology, applied in computational theoretical chemistry, chemical structure search, chemical process analysis/design, etc., can solve the problems of unknown quantum chemical methods, different quantum chemical methods, etc., and achieve low cost , Wide range of application, fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
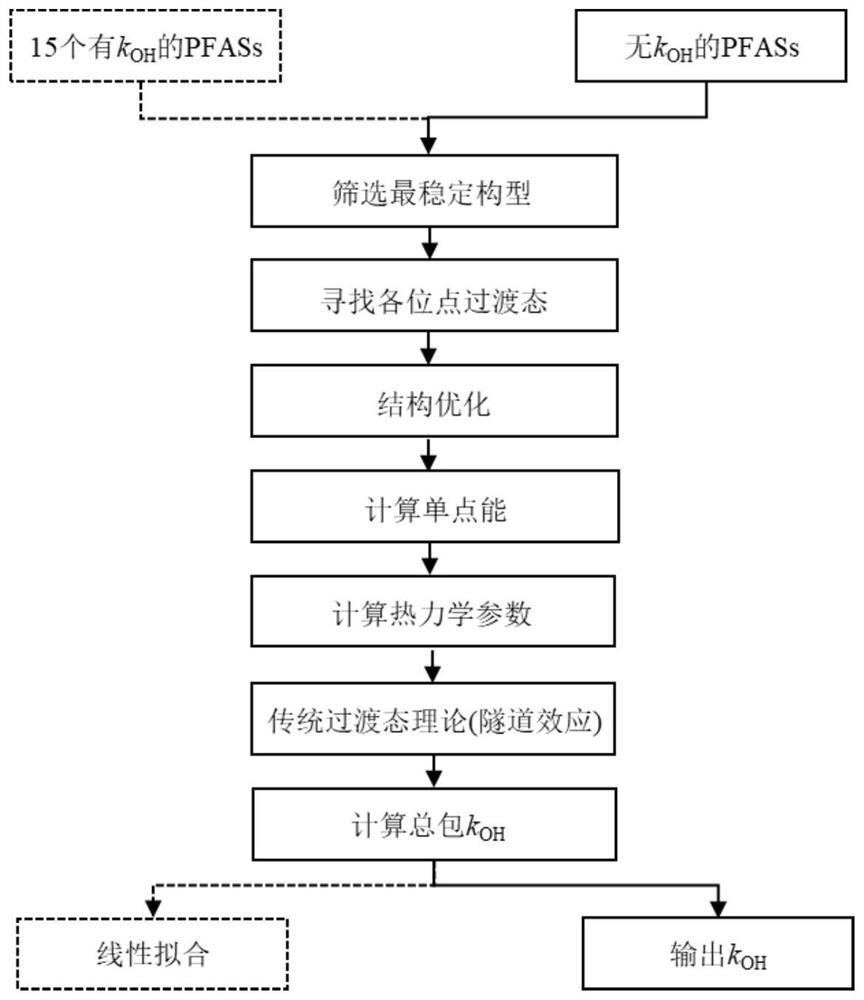
[0048] Select 15 known k OH The PFASs of the PFASs were firstly downloaded for their 3D configuration, and the CP2K software was used to perform BOMD simulation. The CP2K output file had typical representative configurations, and the M06-2X / cc-pVDZ method was used to optimize the structure and obtain its most stable configuration according to the energy. Using this method, the transition states of each channel for the reaction of ·OH with 15 PFASs were also searched.
[0049]
[0050] Refer to the structure and logk of PFASs OH (k OH :cm 3 molecule -1 ·s -1 )
[0051]
[0052] CF 3 CFH 2 (a), CF 3 CH 2 CF 3 (b), C 2 F 5 (CHF) 2 CF 3 (c) Reaction channel for reaction with OH
[0053]
[0054] CH 3 OC 2 F 5 (d), CF 3 OCF 2 H(e), CF 3 CHCl 2 (f) Reaction channel for reaction with OH
[0055]
[0056]
[0057] CF 2 =CH 2 (g), CF 3 OCF=CF 2 (h), CF 3 CF=CF 2 (i), 8:2FTO(j), 4:2FTA(k) reaction channel with OH
[0058]
[0059]
[0...
Embodiment 2
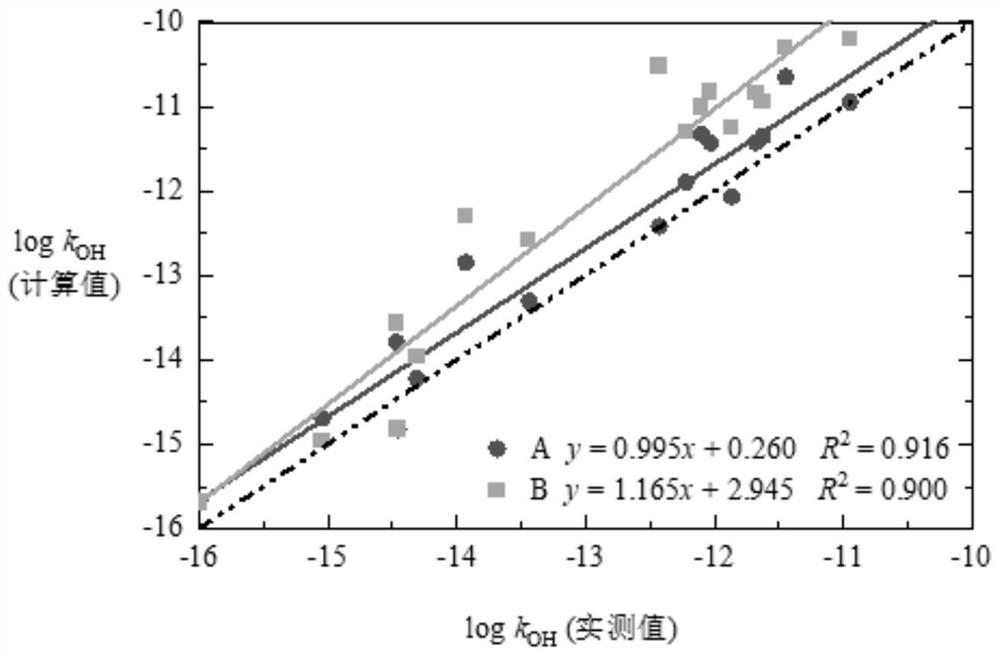
[0084] Given a PFASs: 1,1,1,2,3,3,3-heptafluoropropane, k OH is 1.622×10 -15 cm 3 molecule -1 ·s -1 . First download its 3D configuration, use CP2K software to perform BOMD simulation, and use the M06-2X / cc-pVDZ method to optimize the structure, calculate the energy, and obtain the 3D configuration of its most stable configuration. coordinate. Similarly, the M06-2X / cc-pVDZ method was used to find the transition states of each channel of the reaction between OH and 1,1,1,2,3,3,3-heptafluoropropane. The structure is optimized with the transition state and the thermodynamic parameters are extracted, and the single-point energy is obtained by the addition of M06-2X-D3 / jun-pVTZ:ωb97x-D / jul-pVTZ=0.5:0.5. The single point energy and thermodynamic parameters are added together, as the final thermodynamic parameter ΔG is 50.945kJ mol -1 ;E a is 14.754kJ mol -1 ; For: 1566.752cm -1 ; V is 0.000kJ mol -1 . and apply it to the TST method to calculate k TST , and multiply b...
Embodiment 3
[0086] Given a PFASs: 1,1,2,2,3-pentafluoropropane, k OH is 1.089×10 -14 cm 3 molecule -1 ·s -1 . First download its 3D configuration from PubChem, use CP2K software to perform BOMD simulation, and use the M06-2X / cc-pVDZ method to optimize the structure, calculate the energy, and obtain the most stable structure. 3D coordinates of the type. Similarly, the M06-2X / cc-pVDZ method was used to find the transition states of each channel in the reaction between OH and 1,1,2,2,3-pentafluoropropane. Structural optimization was carried out and thermodynamic parameters were extracted, and the single-point energy was obtained by the summation of M06-2X-D3 / jun-pVTZ:ωb97x-D / jul-pVTZ=0.5:0.5. The single point energy and thermodynamic parameters are added together as the final thermodynamic parameter ΔG for channel 1: 43.2kJ mol -1 , channel 2: 49.3 kJ mol -1 , channel 3: 48.1 kJ mol -1 ;Channel 1:E a 5.634kJ mol -1 , channel 2: E a is 13.126kJ mol -1 , channel 3: E a 9.720kJmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com