Tau protein visual PROTAC degradation compound as well as preparation method and application thereof
A compound and protein technology, applied in the field of Tau protein visualization PROTAC degradation compound and its preparation, can solve the problem of no retention of imaging function, and achieve the effect of strong degradation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1. Condensation of target carboxylic acid intermediate and linker / E3 ligand complex intermediate to obtain PROTAC final product molecule
[0117] Synthetic route of PROTACs using VHL ligand as E3 ligand
[0118]
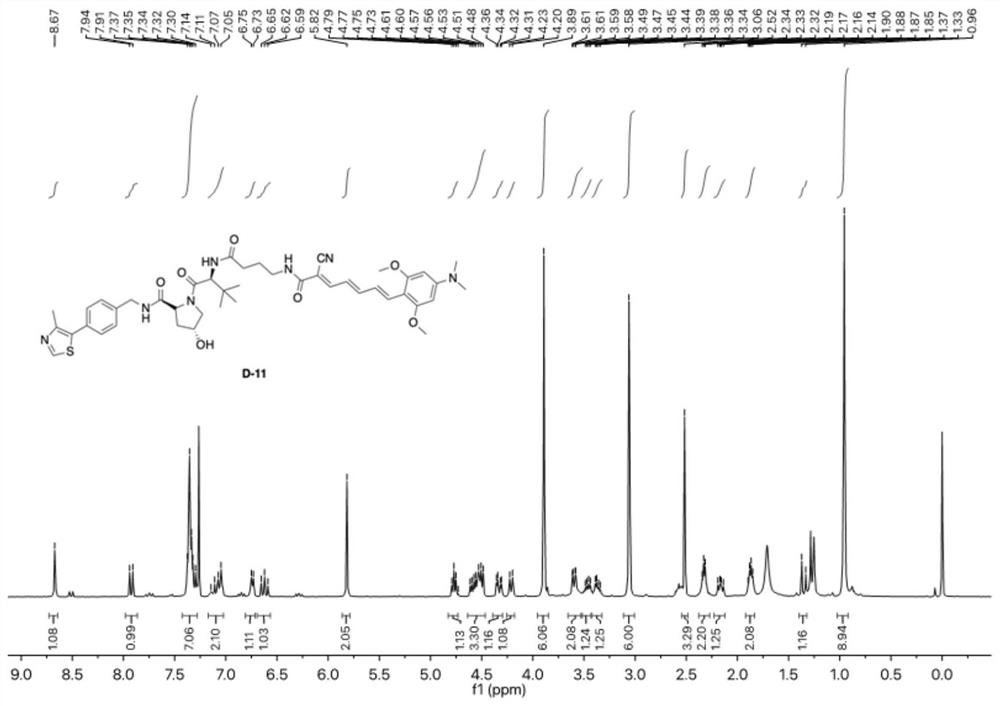
[0119] (2R,4R)-1-((S)-2-(4-((2E,4E,6E)-2-cyano-7-(4-(dimethylamino)-2,6-dimethoxy Phenyl))hept-2,4,6-trienamido)butanamido)-3,3-dimethylbutyryl)-4-hydroxy-N-(4-(4-methylthiazole-5) -yl)benzyl)pyrrolidine-2-carboxamide D-11
[0120] The specific synthesis steps of D-11:
[0121] AD-4-5 (66.00 mg, 0.20 mmol) was dissolved in 7 mL of tetrahydrofuran, DIPEA (105.00 mg, 0.81 mmol) and DMAP (30.00 mg, 0.24 mmol) were added thereto under stirring, and the reaction solution was cooled to 0 °C After stirring for 10 min, HATU (155.00 mg, 0.41 mmol) was added, the temperature was naturally raised to room temperature, AD-L-V-3-2 (103.00 mg, 0.20 mmol) was added in batches after stirring for half an hour, and the mixture was stirred at room temperature for 12...
Embodiment 2
[0126] Example 2. Condensation of target carboxylic acid intermediate and linker / E3 ligand complex intermediate to obtain PROTAC final product molecule
[0127] Synthetic route of PROTACs using VHL ligand as E3 ligand
[0128]
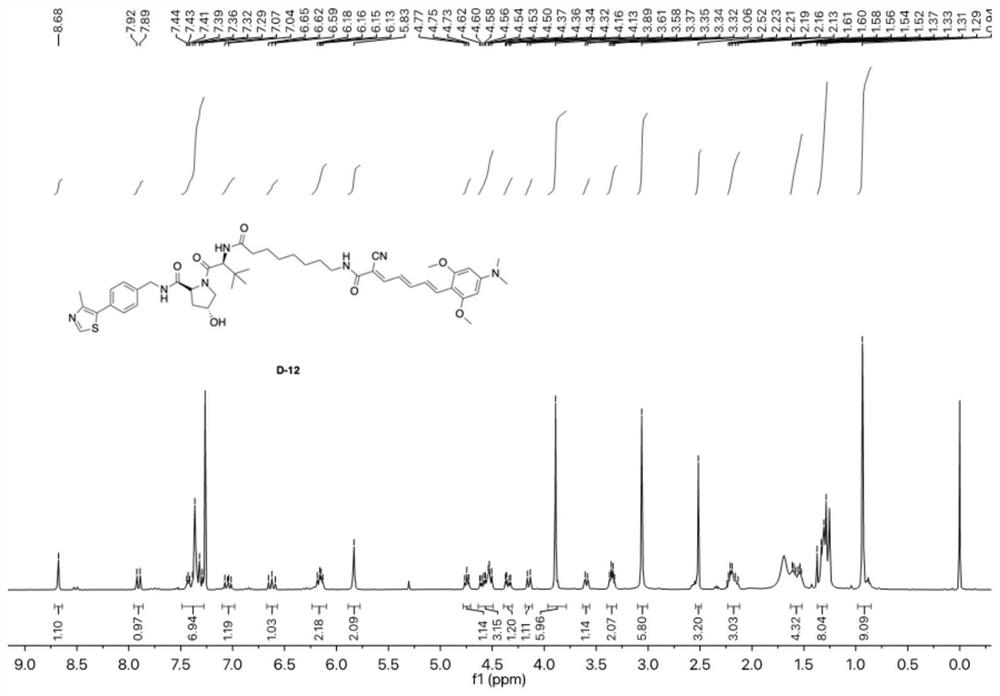
[0129] (2R,4R)-1-((S)-2-(8-((2E,4E,6E)-2-cyano-7-(4-(dimethylamino)-2,6-dimethoxy phenyl))hept-2,4,6-trienamido)octamido)-3,3-dimethylbutyryl)-4-hydroxy-N-)4-(4-methylthiazole- 5-yl)benzyl)pyrrolidine-2-carboxamide D-12;
[0130] The specific synthesis steps of D-12:
[0131] AD-4-5 (66.00 mg, 0.20 mmol) was dissolved in 7 mL of tetrahydrofuran, DIPEA (105.00 mg, 0.81 mmol) and DMAP (30.00 mg, 0.24 mmol) were added thereto under stirring, and the reaction solution was cooled to 0 °C After stirring for 10 minutes, HATU (155.00 mg, 0.41 mmol) was added, the temperature was naturally raised to room temperature, AD-L-V-2-2 (114.00 mg, 0.20 mmol) was added in batches after stirring for half an hour, and the mixture was stirred at room temperature for ...
Embodiment 3
[0137] Example 3. Condensation of target carboxylic acid intermediate and linker / E3 ligand complex intermediate to obtain PROTAC final product molecule
[0138] Synthetic route of PROTACs using pomalidomide as E3 ligand
[0139]
[0140] (2E,4E,6E)-2-cyano-7-(4-(dimethylamino)-2,6-dimethoxyphenyl)-N-(5-((2-(2,6- Dioxypiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)pentyl)hept-2,4,6-trienamide D-16;
[0141] The specific synthesis steps of D-16:
[0142] Dissolve AD-4-5 (66.00 mg, 0.20 mmol) in 7 mL of tetrahydrofuran, add DIPEA (105.00 mg, 0.81 mmol) and DMAP (30.00 mg, 0.24 mmol) to it under stirring, and cool the reaction solution to 0 °C After stirring for 10 minutes, HATU (155.00 mg, 0.41 mmol) was added, the temperature was naturally raised to room temperature, AD-L-B-2-2 (71.00 mg, 0.20 mmol) was added in batches after stirring for half an hour, and the mixture was stirred at room temperature for 12 h. After monitoring the completion of the reaction, 14 mL of water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com