2-trifluoroalkyl-1, 4-naphthoquinone compound and synthesis method thereof
A technology of trifluoroalkyl and synthesis method, which is applied in the field of 2-trifluoroalkyl-1,4-naphthoquinone compounds and their preparation, and achieves the effects of simple operation, low production cost and wide substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
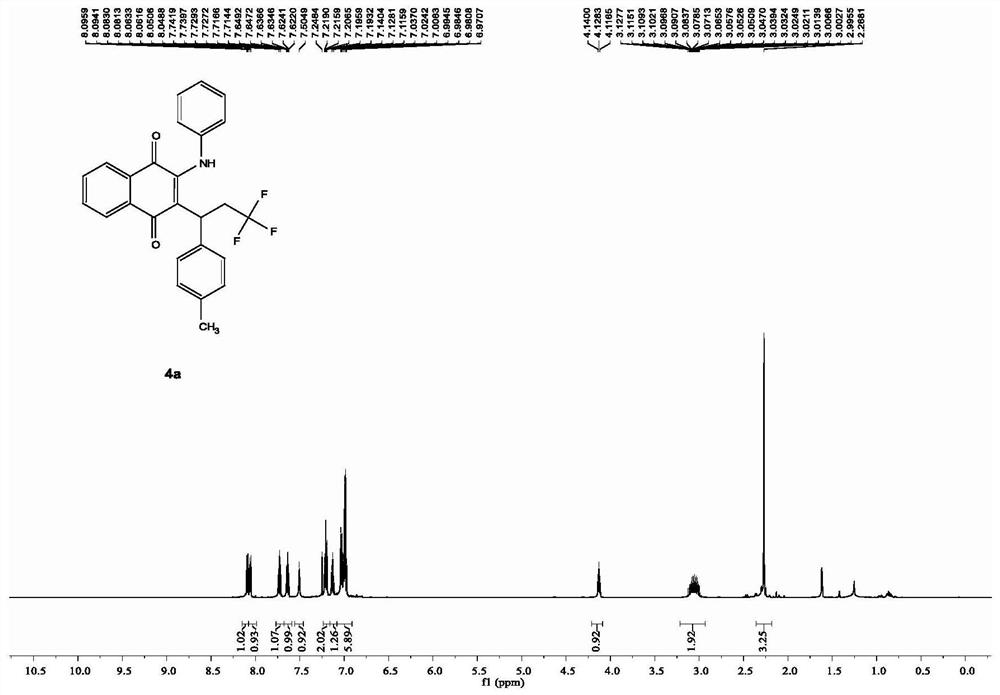
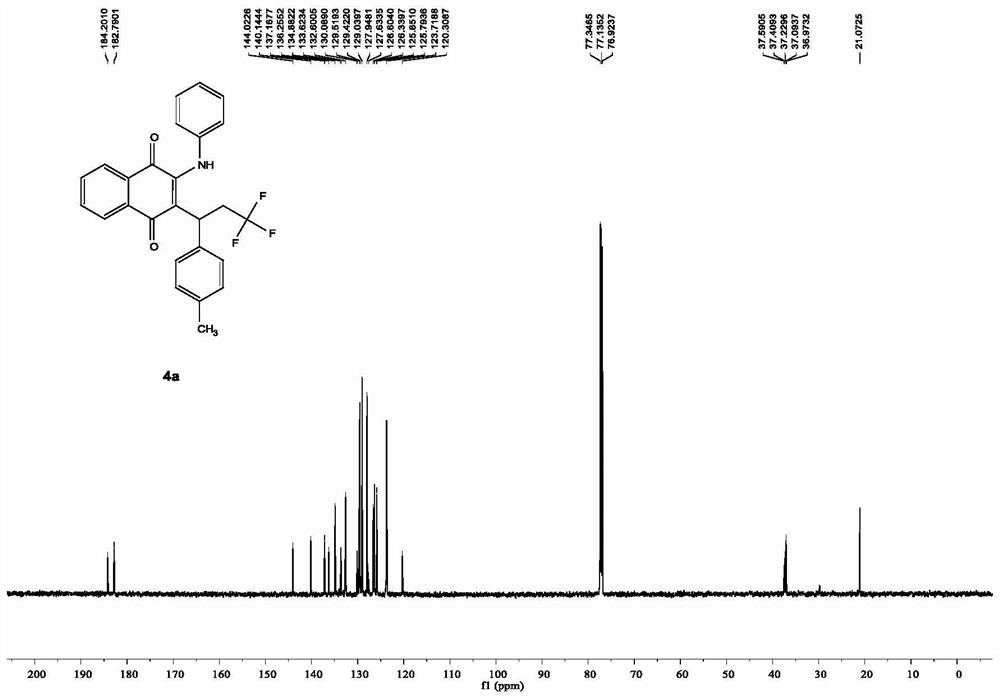
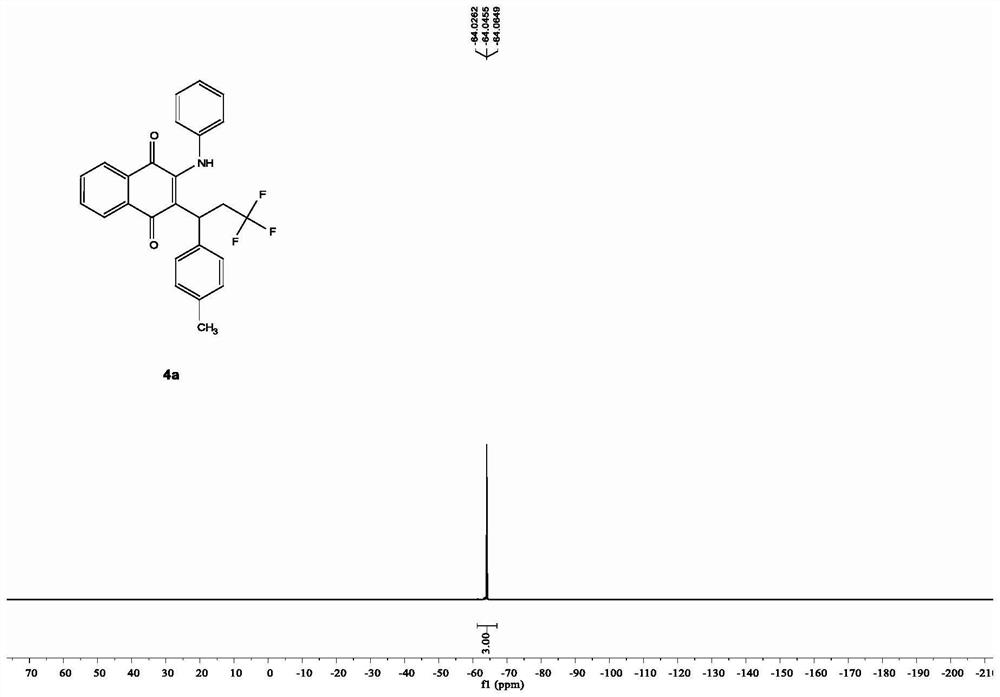
[0022] In a clean 10mL Schlenk pressure reaction tube, add 0.02mmol ferrous sulfate heptahydrate, 0.3mmol p-methylstyrene, 0.2mmol 2-phenylamino-1,4-naphthoquinone, 0.4mmol trifluoromethylidene in turn Sodium sulfonate, 0.5 mmol potassium persulfate, and 2 mL of acetonitrile were added as solvent, the reaction tube was replaced with nitrogen through a double-row tube, and then placed in an oil bath at 100° C. for heating and reaction for 20 hours. After the reaction, the acetonitrile was directly removed by a rotary evaporator, and the obtained residue was separated by a silica gel column using petroleum ether and ethyl acetate as eluents, and the obtained target product was a red solid. Characterization data are: 1 HNMR (600MHz, CDCl 3 ): δ[ppm]=8.10-8.08 (dd, J=7.7, 1.1Hz, 1H), 8.06-8.05 (dd, J=7.8, 1.2Hz, 1H), 7.74-7.71 (td, J=7.7, 1.1 Hz,1H),7.65-7.62(td,J=7.7,1.1Hz,1H),7.50(s,1H),7.22-7.19(t,J=7.8Hz,2H),7.14-7.12(t,J= 7.7Hz, 1H), 7.04-6.97(m, 6H), 4.14-4.12(t, J=7.1Hz,...
Embodiment 2
[0024] In a clean 10mL Schlenk pressure reaction tube, add 0.02mmol ferrous sulfate heptahydrate, 0.3mmol p-tert-butylstyrene, 0.2mmol 2-anilino-1,4-naphthoquinone, 0.4mmol trifluoromethyl in turn Sodium sulfinate, 0.5 mmol potassium persulfate, and 2 mL of acetonitrile were added as a solvent, the reaction tube was replaced with nitrogen through a double-row tube, and then placed in an oil bath at 100° C. for heating and reaction for 20 hours. After the reaction, the acetonitrile was directly removed by a rotary evaporator, and the obtained residue was separated by a silica gel column using petroleum ether and ethyl acetate as eluents, and the obtained target product was a red solid. Characterization data are: 1 H NMR (600MHz, CDCl 3 ): δ[ppm]=8.11-8.09 (dd, J=7.7, 1.1Hz, 1H), 8.07-8.05 (dd, J=7.7, 1.2Hz, 1H), 7.75-7.71 (td, J=7.7, 1.1 Hz,1H),7.65-7.62(td,J=7.8,1.2Hz,1H),7.48(s,1H),7.21-7.14(m,4H),7.11-7.09(t,J=7.7Hz,1H) ,7.01-6.97(m,4H),4.15-4.12(t,J=7.2Hz,1H),3.19-3.10(m...
Embodiment 3
[0026] In a clean 10mL Schlenk pressure reaction tube, add 0.02mmol ferrous sulfate heptahydrate, 0.3mmol p-acetoxystyrene, 0.2mmol 2-anilino-1,4-naphthoquinone, 0.4mmol trifluoromethyl in turn Sodium sulfinate, 0.5 mmol potassium persulfate, and 2 mL of acetonitrile were added as a solvent, the reaction tube was replaced with nitrogen through a double-row tube, and then placed in an oil bath at 100° C. for heating and reaction for 20 hours. After the reaction, the acetonitrile was directly removed by a rotary evaporator, and the obtained residue was separated by a silica gel column using petroleum ether and ethyl acetate as eluents, and the obtained target product was a red solid. Characterization data are: 1 H NMR (600MHz, CDCl 3 ): δ[ppm]=8.09-8.06(m, 2H), 7.75-7.73(td, J=7.7, 1.1Hz, 1H), 7.66-7.63(td, J=7.7, 1.2Hz, 1H), 7.57( s,1H),7.20-7.18(t,J=7.8Hz,2H),7.13-7.11(t,J=7.8Hz,1H),7.04-7.03(d,J=8.5Hz,2H),7.00-6.99 (d,J=8.5Hz,2H),6.89-6.87(d,J=8.6Hz,2H),4.11-4.09(t,J=7.1Hz,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com