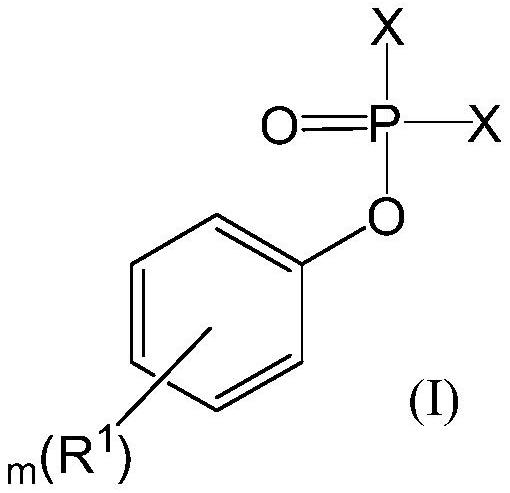
Preparation method of aryloxy phosphorylated amino-acid ester compound
A technology for aryloxy phosphorylation of amino acid esters and compounds, which is applied in the field of medicine, can solve the problems of high production equipment requirements, high production costs, and high reactivity, and achieve large-scale production, increase total yield, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0120] These examples are not provided to limit the scope of the invention, but merely to illustrate various elements of the invention in greater detail.
[0121] The structures of the compounds were determined by NMR spectroscopy ( 1 H NMR) and / or mass spectrometry (MS) for confirmation.
[0122] Chemical shifts (δ) are given in parts per million (ppm). 1 The determination of H NMR was carried out on a Bruker 400 or Bruker 600 or Varian 300 nuclear magnetic instrument, and the test solvent was deuterated methanol (CD). 3 OD), deuterated chloroform (CDCl 3 ) or hexadeuterated dimethyl sulfoxide (DMSO-d 6 ), the internal standard is tetramethylsilane (TMS).
[0123] LC-MS was measured on Agilent LC-MS-1110 LC / MS, Agilent LC-MS-6110 LC / MS, Agilent LC-MS-6120 LC / MS (manufacturer: Agilent) or Shimadzu LC - On MS 2020.
[0124] Preparative high performance liquid chromatography was performed using an MS-triggered automated purification system (Waters), a Gilson GX-281 (Gilson...
Embodiment 2
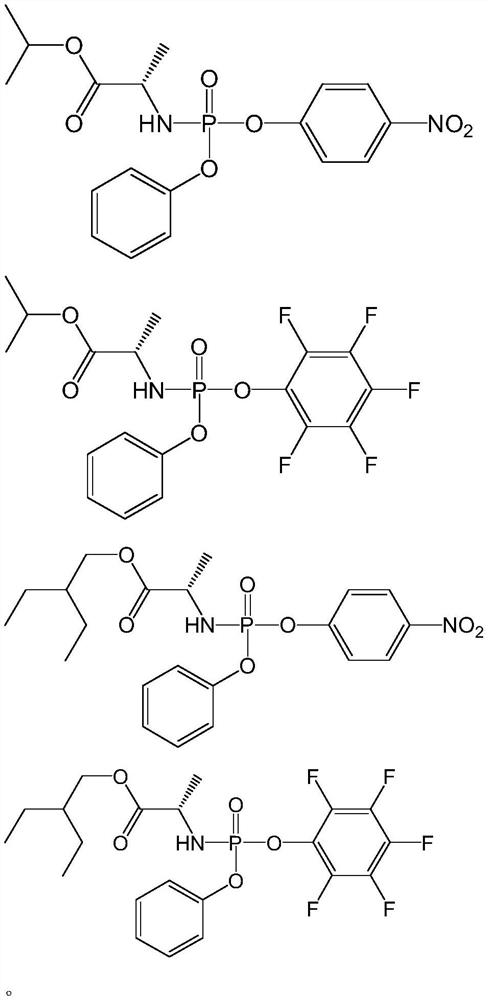
[0132] Example 2: Preparation of [(2s)-(2,3,4,5,6-pentafluoro-phenoxy)-phenoxy-phosphoryl]-L-alanine isopropyl ester
[0133]
[0134] The method is the same as in Example 1, except that L-alanine (2-ethylbutyl) ester in the raw material is replaced with L-alanine isopropyl ester 1.312g (10.0mmol) to obtain the target product [(2s)-( 2,3,4,5,6-Pentafluoro-phenoxy)-phenoxy-phosphoryl]-L-alanine isopropyl ester.
[0135] its nuclear magnetic 1 H NMR (600MHz, CDCl 3 ):δ1.25(t,J=7.8Hz,6H),1.46(d,J=7.2Hz,3H),3.94-4.00(m,1H),4.01(t,J=10.2Hz,2H),4.11 -4.17(m,1H), 5.02-5.06(m,1H), 7.20-7.37(m,5H); NMR 13 C NMR (125MHz, CDCl 3 ): δ172.48, 172.42, 150.17, 150.12, 129.83, 125.63, 120.06, 120.03, 69.60, 50.61, 21.62, 21.59, 20.96, 20.93; NMR 31 P NMR (240MHz, CDCl 3 ): δ-1.59; high resolution mass spectrometry HRMS (ESI) calcd.for C 18 H 18 F 5 NO 5 P + [M+H] + :454.0837,found:454.0819.
Embodiment 3
[0136] Example 3: Preparation of [(2s)-(4-nitro-phenoxy)-phenoxy-phosphoryl]-L-alanine (2-ethylbutyl) ester
[0137]
[0138] The method is the same as in Example 1, except that 2,3,4,5,6-pentafluorophenol in the raw material is replaced by 1.391g (10.0mmol) of 4-nitrophenol to obtain the target product [(2s)-(4-nitrophenol) -Phenoxy)-phenoxy-phosphoryl]-L-alanine (2-ethylbutyl) ester.
[0139] its nuclear magnetic 1 H NMR (600MHz, CDCl 3 ):δ0.87(t,J=7.2Hz,6H),1.29-1.35(m,4H),1.41(d,J=7.2Hz,3H),1.47-1.53(m,1H),3.97(dd, J=11.4, 9.6Hz, 1H), 4.04(qd, J=10.8, 6.0Hz, 2H), 4.13-4.19(m, 1H), 7.19-7.24(m, 3H), 7.35(t, J=7.8Hz ,2H),7.40(d,J=9.0Hz,2H); NMR 13 C NMR (125MHz, CDCl 3 ): δ173.17,173.11,155.60,155.56,150.23,150.19,144.67,129.88,125.64, 125.53,120.82,120.78,120.13,120.10,67.84,50.54,50.52,45.79,40.23, 23.18,23.15,21.16,21.13,10.96, 10.93, 8.63; NMR 31 P NMR (240MHz, CDCl 3 ): δ-3.16; high resolution mass spectrometry HRMS (ESI) calcd.for C 21 H 28 N 2 O 7 P + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com