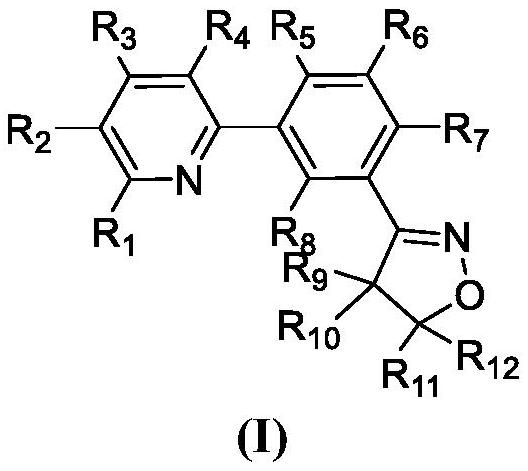
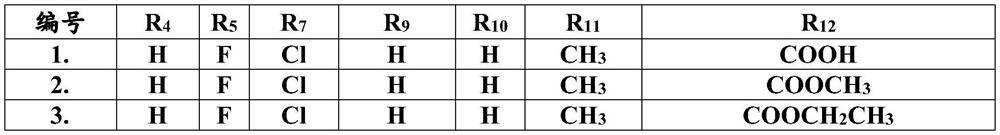
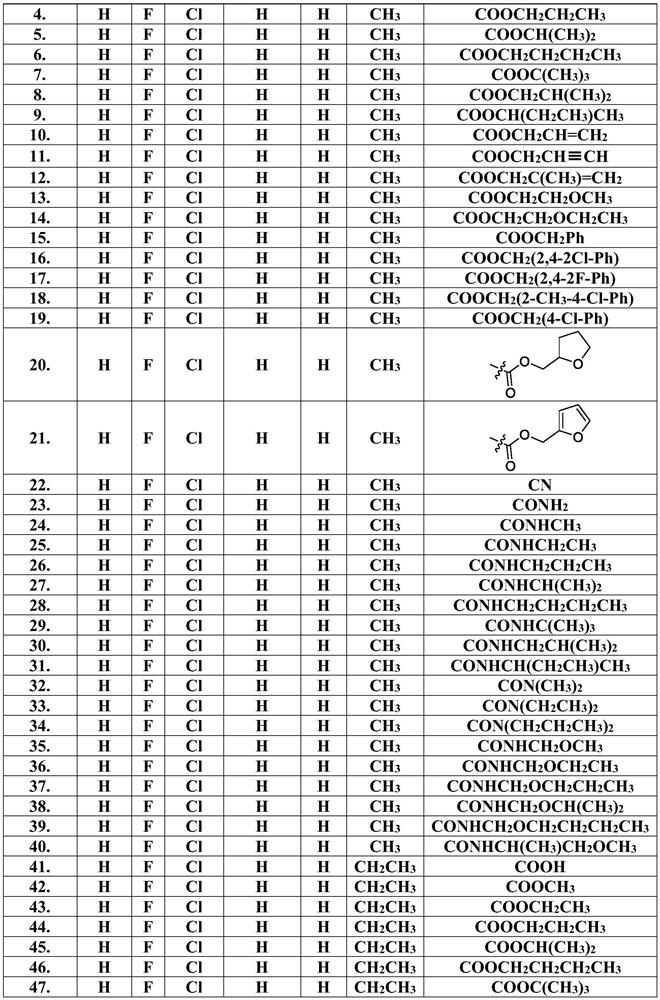
Pyridine biphenyl compound containing isoxazoline as well as preparation method and application of pyridine biphenyl compound
A kind of technology of compound and solvate, applied in the field of pyridine biphenyl compounds of isoxazoline and preparation thereof, can solve problems such as satisfactory herbicidal performance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] Example 1: Ethyl 3-(2-chloro-4-fluoro-5-(5-(trifluoromethyl)pyridin-2-yl)phenyl)-5-methyl-4,5-dihydroiso Preparation of oxazole-5-carboxylate (compound 3)
[0214]
[0215] The first reaction: the preparation of 2-chloro-4-fluoro-5-(5-(trifluoromethyl)pyridin-2-yl)benzaldehyde: under nitrogen protection, 3.03g (0.015mol) (4- Chloro-2-fluoro-5-formylphenyl)boronic acid, 6.21g (0.045mol) potassium carbonate, 0.52g (0.00045mol) tetrakis (triphenylphosphine) palladium, 60ml tetrahydrofuran, 30ml water were added to the there-necked flask and stirred . To the above mixture was added portionwise 3.27 g (0.018 mol) of 2-chloro-5-(trifluoromethyl)pyridine. After the addition was completed, the reaction was stirred at 60°C for 8 hours. The reaction solution was cooled to room temperature. Desolvate under reduced pressure. The residue was extracted with ethyl acetate (2*20ml) and the organic phases were combined. Wash once. Wash once with saturated salt water. Dry over...
Embodiment 2
[0222] Example 2: Methyl 3-(2-chloro-5-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)-4-fluorophenyl)-5-methyl-4,5 - Preparation of dihydroisoxazole-5-carboxylate (compound 122)
[0223]
[0224]The first step reaction: preparation of 2-chloro-5-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)-4-fluorobenzaldehyde: under nitrogen protection, 3.03g (0.015mol ) (4-chloro-2-fluoro-5-formylphenyl)boronic acid, 6.21g (0.045mol) potassium carbonate, 0.52g (0.00045mol) tetrakis(triphenylphosphine)palladium, 60ml tetrahydrofuran, 30ml water were added to the Stir in a three-necked bottle. To the above mixture was added portionwise 3.89 g (0.018 mol) of 2,3-dichloro-5-(trifluoromethyl)pyridine. After the addition was completed, the reaction was stirred at 60°C for 8 hours. The reaction solution was cooled to room temperature. Desolvate under reduced pressure. The residue was extracted with ethyl acetate (2*20ml) and the organic phases were combined. Wash once. Wash once with saturated ...
Embodiment 3
[0231] Example 3: Ethyl 3-(2-chloro-5-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)-4-fluorophenyl)-5-methyl-4,5 - Preparation of dihydroisoxazole-5-carboxylate (compound 123)
[0232]
[0233] At room temperature, 1.77g (0.005mol) 2-chloro-5-(3-chloro-5-trifluoromethyl)pyridin-2-yl)-4-fluorobenzaldehyde oxime, 1.01g (0.0075mol) N- Chlorosuccinimide and 10 ml of N,N-dimethylformamide were added to the three-necked flask, and the reaction was stirred at 40° C. for 2 hours. The reaction solution was cooled to room temperature, 0.68g (0.006mol) of ethyl methacrylate, 0.76g (0.0075mol) of triethylamine in 2ml of N,N-dimethylformamide solution were added to the above mixture, and the mixture was stirred at room temperature for 5 hours . The reaction mixture was diluted with water and ethyl acetate and the phases were separated. The aqueous phase was extracted with ethyl acetate (2*20ml) and the organic phases were combined. The organic phase was washed twice with water and once...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com