Method for preparing methylacrolein and co-producing methacrylonitrile by oxidizing isobutene
A technology of methacrolein and methacrylonitrile, applied in the direction of hydrocarbon ammoxidation preparation, carbon-based compound preparation, chemical instruments and methods, etc., can solve the problems of methacrylonitrile strength, achieve co-production, solve strong Response heat problem, effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation process of the catalyst used in the following embodiment is as follows:
[0062] 31.8g ammonium heptamolybdate was added to 60mL deionized water, and then 20mL of ammonia water was added, at this time the solution was clarified, 18.16g 30wt% silica sol was added under mechanical stirring, and the above mixture was placed in an 80 ° C water bath pot. 1.51g of potassium nitrate solution with a mass fraction of 10%, 17.44g of nickel nitrate hexahydrate, 7.7g of magnesium nitrate hexahydrate, 15.17g of iron nitrate hexahydrate, 1.82g of bismuth pentahydrate, 4.47g of zinc nitrate hexahydrate, 10.88g of copper trihydrate nitrate dissolved in 30g of deionized water and then added 1g of citric acid, stirred to dissolve, and slowly added to the ammonium heptamolybdate solution. After that, continue stirring for 2 hours, transfer the mixture to a 110 °C oven and dry overnight. The dried catalyst is ground, placed in a muffle furnace, in an air atmosphere, at a heatin...
Embodiment 1-4
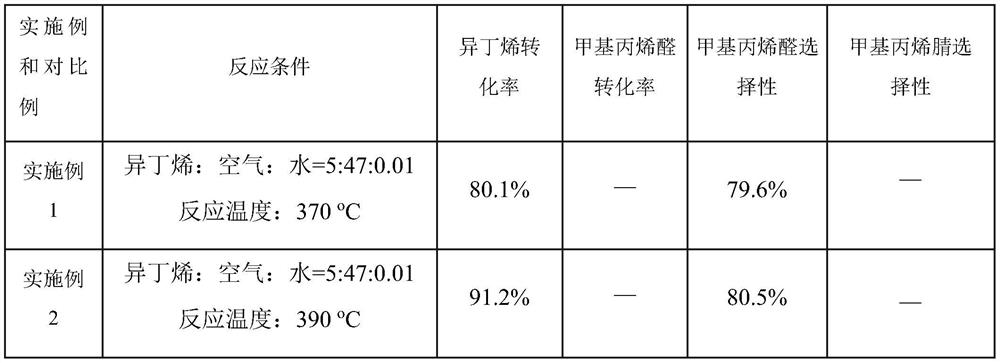
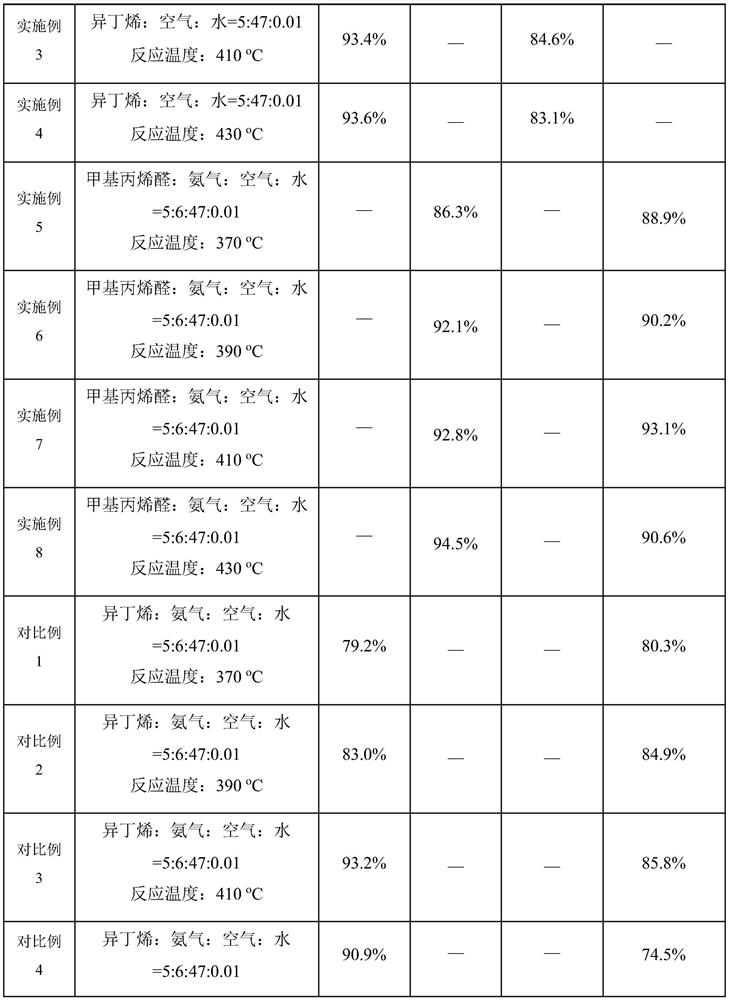
[0065] The 10g catalyst was loaded into a reaction tube with an inner diameter of 16mm in a fixed-bed reactor, and isobutene, air, and water were introduced into the reaction tube, isobutene: air: water = 5: 47: 0.01 (unit mL / min), the reaction conditions were 370-430 °C, atmospheric pressure, and methacrylaldehyde was prepared.
Embodiment 5-8
[0067] Example 1, 2, 3, 4 prepared to obtain methacryl, to the reaction tube and then into the ammonia, methacrylal: ammonia: air: water = 5: 6: 47: 0.01 (unit mL / min), the reaction conditions are 370-430 °C, atmospheric pressure, preparation of methacrylonitrile.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com