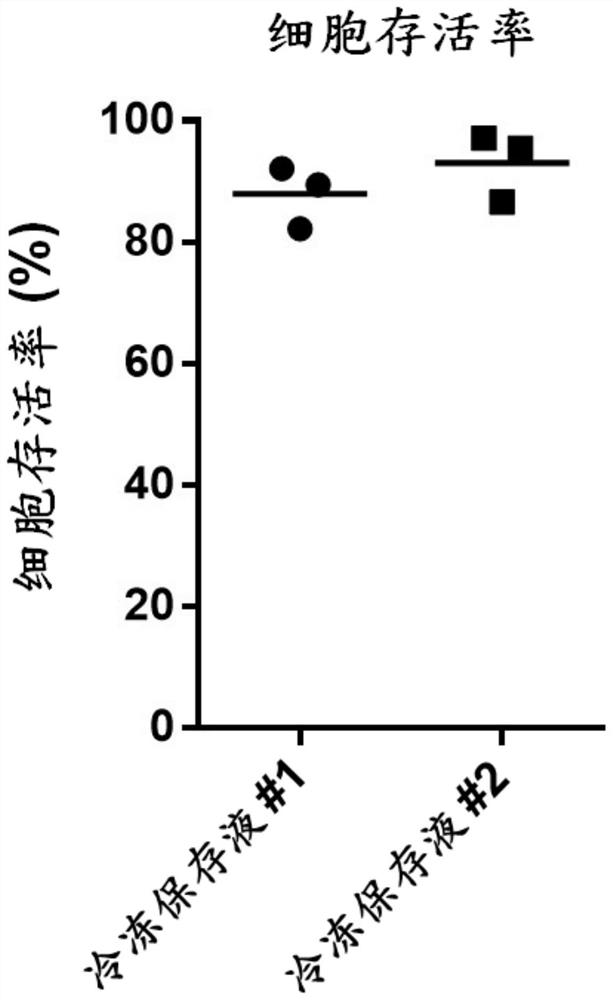
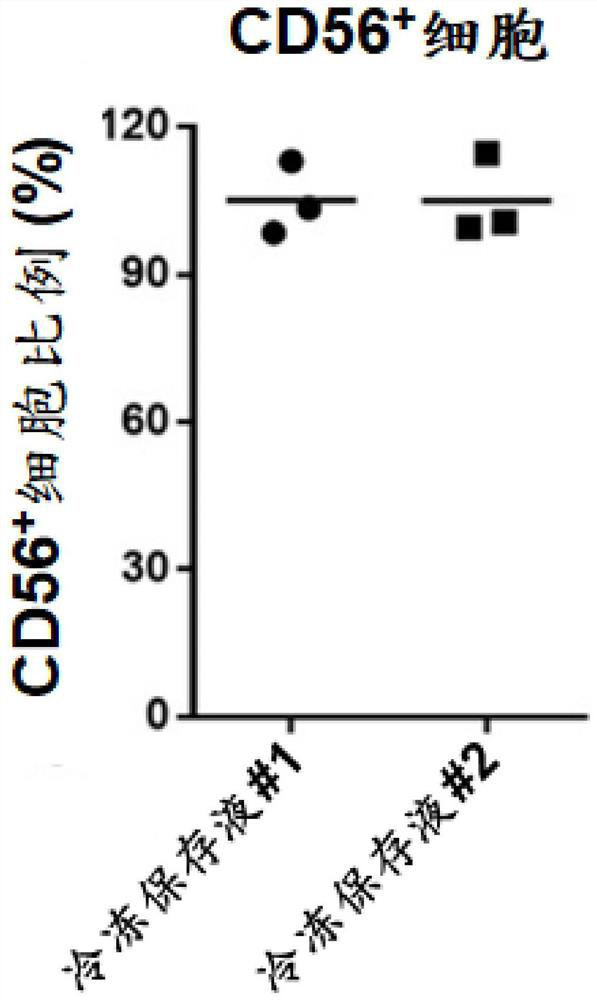
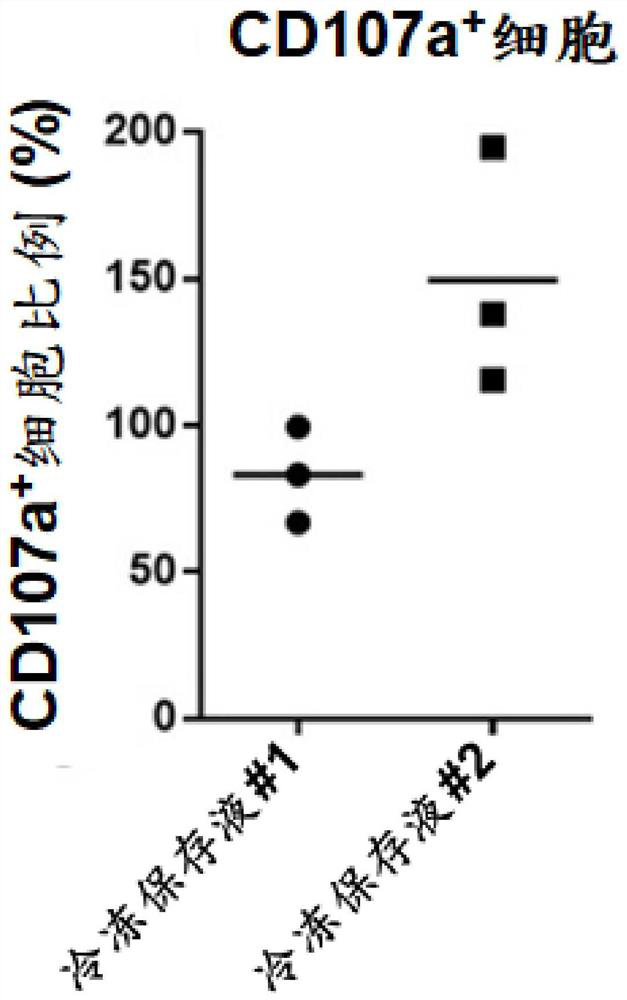
Serum-free cell cryopreservation liquid for cell cryopreservation and cryopreservation method thereof
A cryopreservation solution and cell technology, applied in the field of serum-free cell cryopreservation solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]Example 1 Cryopreservation solution preparation
[0033] In a sterile operating environment, mix the mixed medium containing Roswell Park Memorial Institute medium, stem cell growth medium or AIM-V medium in an unequal ratio of 30 to 70 wt%.
[0034] In an aseptic operating environment, polymers containing dextran are configured on a mixed medium basis. Among them, the molecular weight of dextran (Dextran) is 40~78.5 kDa, and the concentration of the prepared stock solution is 20% (V / V). Different proportions of the stock concentrations are added to the cryopreservation solution, and the final concentration is between 5 and 10% (V / V).
[0035] In a sterile working environment, dimethyl sulfoxide was added to cryopreservation solution, and the final concentration ranged from 2.5 to 10% (V / V).
[0036] In a sterile operating environment, platelet lysate is added to cryopreservation solution, and the final concentration ranges from 1 to 10% (V / V).
[0037] Pre-cool the configur...
Embodiment 2
[0039] Example 2 Cell cooling freeze
[0040] Cells to be frozen are washed at 1×10 6 ~1×10 8 The cells are suspended at / mL cell concentration in cryopreservation solution and aliquoted in cryovials.
[0041] Refrigerated tubules containing the cell suspension are placed in a commercially available cell cryo-box (CoolCell LX, Corning) and the cryophile is placed in an ultra-low temperature freezer at -80°C for 8 to 16 hours. The next day pipettes that have been cooled are pipetted into liquid nitrogen drums for storage.
[0042] In addition to using the cell cryostat, you can also use the microcomputer program cooler (IceCube 14S, SY-LAB) for full temperature control and cooling. After the cooling is completed, it can be directly transferred to the liquid nitrogen drum for preservation.
Embodiment 3
[0043]Example 3 Cell thawing
[0044] Pipette the cryovials out of the liquid nitrogen drum and place them in a pre-cooled alloy metal heat pipe holder (CoolRack CF15, Corning) throughout the transfer process. After the cells are completely thawed in a 37 °C water bath, follow-up experiments or culture steps are performed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com