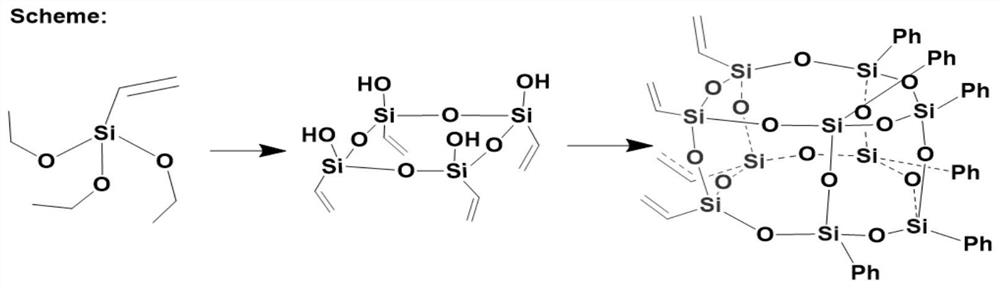
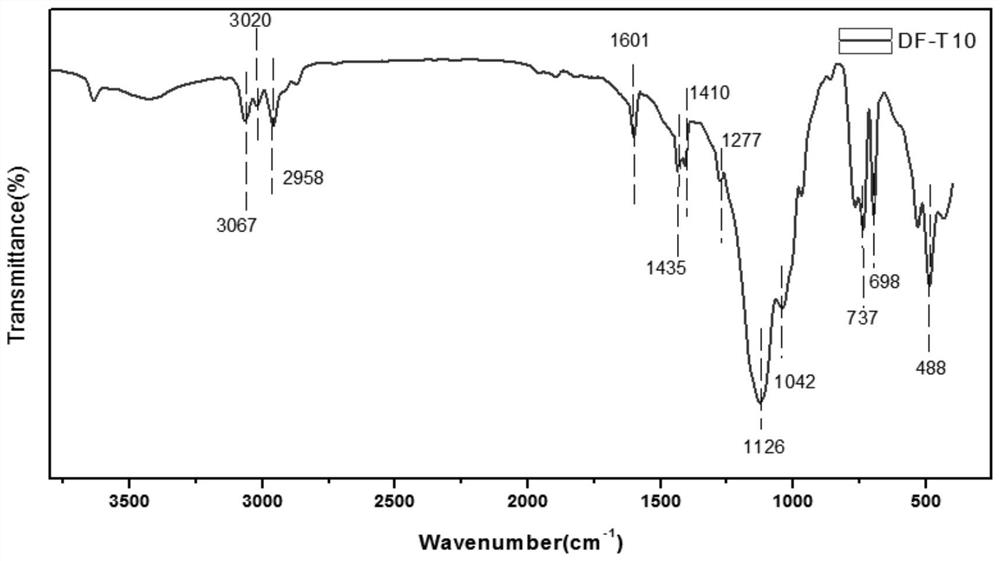
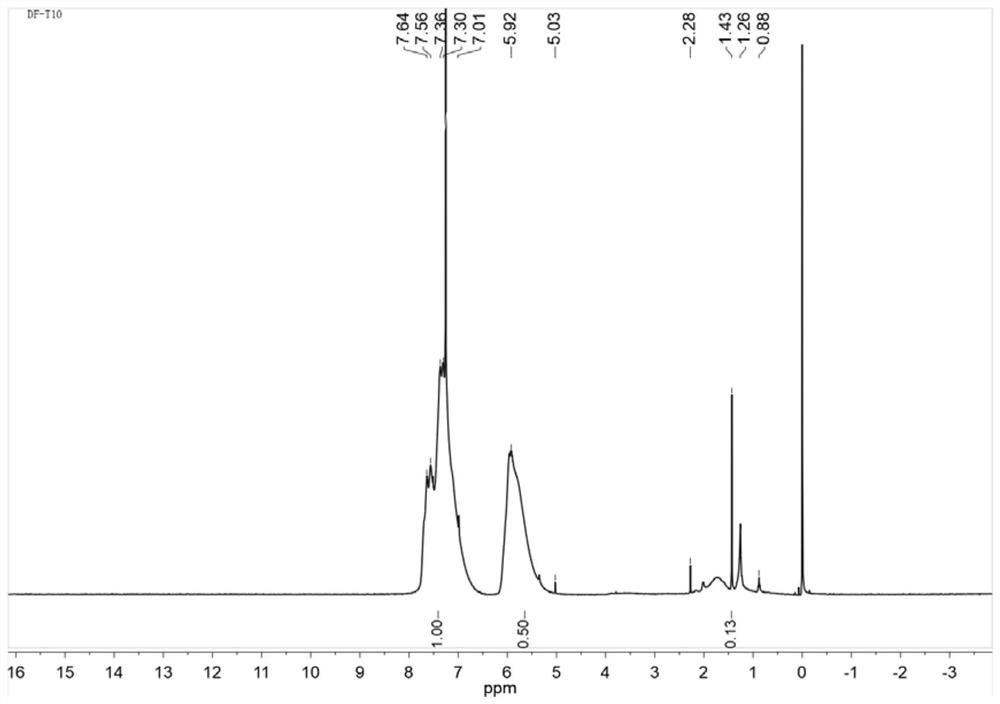
Tetraethylene hexaphenyl bifunctional group-containing T10 polyhedral oligomeric silsesquioxane and preparation method thereof
A technology of tetraethylene hexaphenyl bifunctional and vinyl triethoxy siloxane is applied in the field of organic-inorganic nano-hybrid materials, and can solve the problems of low yield, difficulty in separation and purification, low yield of target product and the like, To achieve the effect of simple reaction process, simple post-processing and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 7g CH 3 OK (100mmol) was packed into a 250ml single-necked flask, 90ml of isopropanol was added, 1.8ml of H 2 O (100mmol), the reaction system is white and turbid; 19g (100mmol) vinyltriethoxysilane and 20ml isopropanol that have been added to the constant pressure funnel are slowly added dropwise to the flask, about 10min, and react at room temperature for 24h; continue Add conc. T 4 (as shown in formula I):
[0033]
[0034] (2) The T obtained in step (1) 4 Take 3.5g (10mmol) and 100ml 1,4-dioxane in a 500ml three-necked flask, add 2.3g potassium methoxide and 2g water, stir to dissolve, and pass through N 2 , then slowly add 12.3g (62mmol) of phenyltrimethoxysilane (PTMS) that has been added in the constant pressure funnel into the flask, and at the same time add the catalyst tetramethylammonium fluoride 0.14g (0.146mmol), after the dropwise addition is completed React at 40°C for 24 hours, and then add anhydrous calcium chloride as a catalyst for rem...
Embodiment 2
[0037] (1) Add 7g CH 3 OK (100mmol) was packed into a 250ml single-necked flask, 90ml of isopropanol was added, 1.8ml of H 2 O (100mmol), the reaction system is white and turbid; 19g (100mmol) vinyltriethoxysilane and 20ml isopropanol that have been added to the constant pressure funnel are slowly added dropwise to the flask, about 10min, and react at room temperature for 24h; continue Add conc. T 4 ;
[0038] (2) The T obtained in step (1) 4Take 3.5g (10mmol) and 100ml 1,4-dioxane in a 500ml three-necked flask, add 2.3g potassium methoxide and 2g water, stir to dissolve, and pass through N 2 , then slowly add 14.9g (62mmol) of phenyltriethoxysilane (PTES) that has been added in the constant pressure funnel into the flask, and add catalyst trihydrate tetrabutylammonium fluoride 0.063g (0.2mmol) at the same time, drop After the addition was completed, react at 40°C for 24 hours, and then add anhydrous calcium chloride as a catalyst for removing fluoride ions at room temper...
Embodiment 3
[0040] (1) Add 7g CH 3 OK (100mmol) was packed into a 250ml single-necked flask, 90ml of isopropanol was added, 1.8ml of H 2 O (100mmol), the reaction system is white and turbid; 19g (100mmol) vinyltriethoxysilane and 20ml isopropanol that have been added to the constant pressure funnel are slowly added dropwise to the flask, about 10min, and react at room temperature for 24h; continue Add conc. T 4 ;
[0041] (2) The T obtained in step (1) 4 Take 3.5g (10mmol) and 100ml 1,4-dioxane in a 500ml three-necked flask, add 2.3g potassium methoxide and 2g water, stir to dissolve, and pass through N 2 , then slowly add 15.6g (65mmol) of phenyltriethoxysilane (PTES) that has been added in the constant pressure funnel into the flask, and add the catalyst benzyltrimethylammonium fluoride 0.25g (1.5mmol) at the same time, drop After the completion of the addition, react at 60°C for 24 hours, then add anhydrous calcium chloride as a catalyst for removing fluoride ions at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com