Induction method of immature oocytes and preparation method of mature oocytes
An oocyte and immature technology, which is used in the induction of immature oocytes and the production of mature oocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] (construction of the carrier)
[0185]From the CAG-DD-hTFAP2C plasmid (Reference 6: "Kobayashi T et al., "Principles of early human development and germ cell program from conserved model systems.", Nature, Vol.546, No.7658, p416-420, 2017 .”) The CAG promoter and destabilization domain (DD) were cloned and inserted into the previously used PiggyBAC vector (reference 7: “Shimamoto S et al., “Hypoxia induces the dormant state in oocytes through expression of Foxo3” , PNAS, https: / / doi.org / 10.1073 / pnas.1817223116, 2019.”), the PB-CAG-DD vector was produced. Next, the cDNAs of eight genes, FIGLA, NOBOX, SOHLH1, LHX8, SUB1, STAT3, TBPL2 and DYNLL1, were amplified from the cDNA of the ovary of a female mouse 13.5 days after fertilization, and the In-Fusion HD cloning kit (Bao Biological company) cloned into the PB-CAG-DD vector. Amplification of each cDNA was carried out by PCR using KOD Fx Neo or KOD Plus Neo DNA polymerase (manufactured by TOYOBO Corporation) according to...
Embodiment 2
[0196] Using mouse iPS cells, induction of differentiation into oocytes was studied in the same manner as ES cells.
[0197] (transfection of vector)
[0198] As iPS cells, mouse BVSC iPS cells prepared using the virus buster established in the paper of Non-Patent Document 1 were used. Using Lipofectamine 2000, the PB-CAG-DD vector containing 8 genes constructed in Example 1 and the hyperactive PBase (hypBase) plasmid were simultaneously transfected into mouse BVSC iPS cells. After culturing at 37°C for 5 days in a serum-free medium supplemented with 2i and LIF to proliferate, a single colony was obtained by selection with puromycin.
[0199] (Induction of differentiation into immature oocytes)
[0200] The iPS cells selected with puromycin were cultured at 37° C. for 5 days in the same manner as in Example 1, thereby inducing differentiation into immature oocytes.
[0201] (production of mature oocytes)
[0202] Next, in the above (transfection of the vector), after the t...
Embodiment 3
[0205] (Identification of Factors Important in Oocyte Formation Genes)
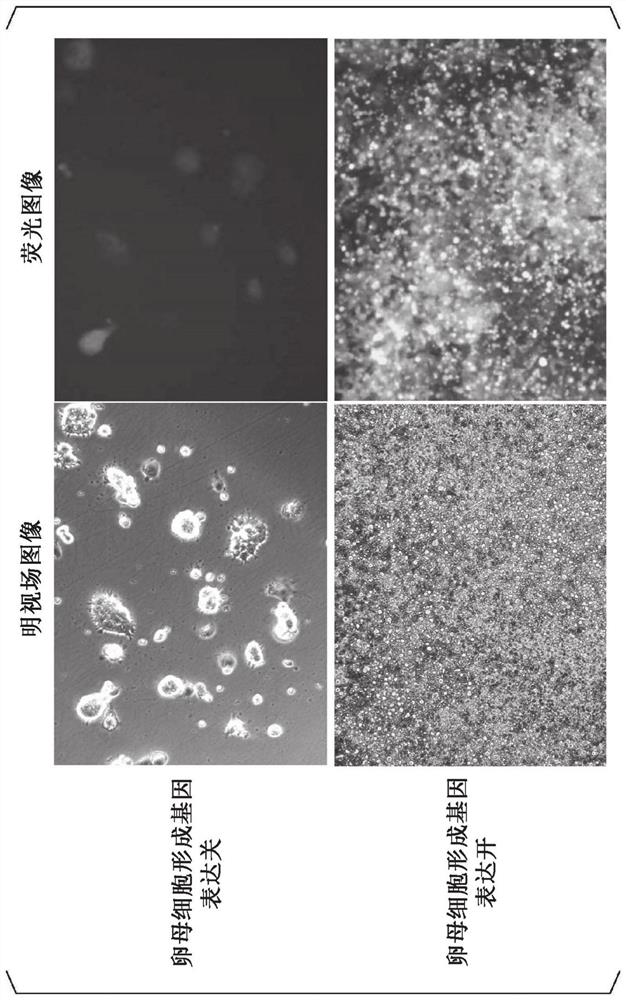
[0206] In order to identify genes that are important factors among the oocyte formation genes to form Figure 4 A total of 26 types of vectors were constructed using the same method as in Example 1 in the manner of combining the genes described in the left figure. Next, each vector was transfected into mouse ES cells (Blimpl-mVenus:Stella-ECFP:Npm2-mCherry(BVSCNmC)) by the same method as in Example 1. After transfection, it was cultured at 37°C for 5 days to proliferate, and ES cells (1×10 5 each) in the above-mentioned oocyte differentiation induction medium with 3×10 4 Ovary somatic cells derived from 12.5-day-old female mice after fertilization were mixed to make agglomerates and cultured at 37°C for 2 days. Next, the obtained aggregate was cultured for 21 days by the same method as in Example 1. For the cultured cells, the number of oocytes formed from each cell line was counted. Furthermore, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com