CDKs inhibitor of beta-carboline parent nucleus and preparation method and anti-tumor application of CDKs inhibitor
A carboline and reaction technology, which is applied in the field of organic compound synthesis and pharmaceutical applications, can solve the problems of neutropenia, anemia or diarrhea, etc., and achieve the goal of inhibiting tumor-bearing growth, simple synthesis method, and inhibiting tumor cell migration and invasion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
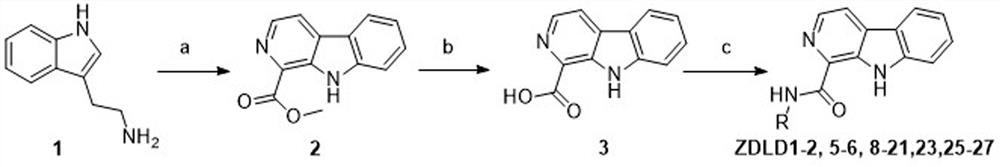
[0035] Methyl 9H-pyrido[3,4-b]indole-1-carboxylate (compound 2)
[0036] In a 500ml round bottom flask was added tryptamine (compound 1, 3g, 18.72mmol), methyl glycolate (2.53g, 28.09mmol), IBX (15.73g, 56.17mmol) and 200ml ethyl acetate. After reflux for 36h, filter. The filtrate was concentrated and purified by column chromatography, and the crude product was recrystallized from PE / EA to give 2 as a yellow solid (36% yield). 1H NMR (600MHz, DMSO-d6) δ11.65(s, 1H), 8.48(d, J=4.9Hz, 1H), 8.41(d, J=4.9Hz, 1H), 8.30(d, J=7.8Hz ,1H),7.79(d,J=8.2Hz,1H),7.63–7.59(m,1H),7.30(t,J=7.5Hz,1H),4.02(s,3H).ESI-MS:m / z 227.1[M+H] + .
Embodiment 2
[0038] 9H-pyrido[3,4-b]indole-1-carboxylic acid (compound 3)
[0039] Compound 2 (0.9g, 4mmol) was added in a 50ml round bottom flask, and 15ml of hydrolyzate was added (hydrolyzate composition: C 2 h 5 OH 175ml, H 2 O 75ml, NaOH 15g). Reflux at 80°C for 1 hour, cool, add 2 mmol / L hydrochloric acid aqueous solution with stirring in an ice bath, and adjust the pH value to 3. Suction filtration gave a yellow solid (compound 3) (yield 96.89%). 1 H NMR (600MHz, DMSO-d 6 )δ11.84(s,1H),8.48(d,J=5.2Hz,1H),8.44(d,J=5.2Hz,1H),8.34(d,J=7.9Hz,1H),7.83(d, J=8.3Hz,1H),7.65–7.60(m,1H),7.34–7.29(m,1H).ESI-MS:m / z 213.1[M+H] + .
Embodiment 3
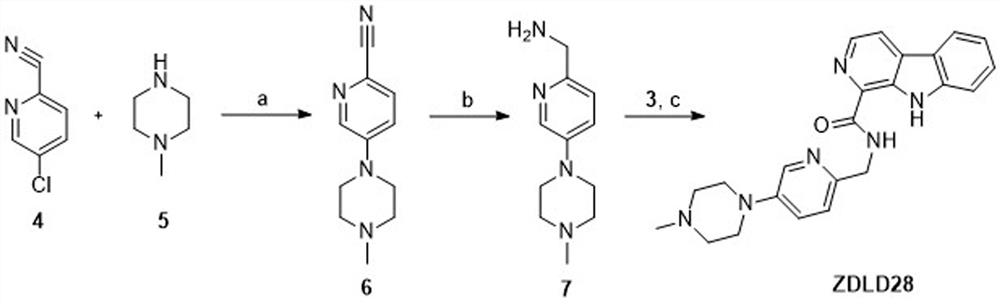
[0041] 5-(4-Methylpiperazin-1-yl)pyridinecarbonitrile (Compound 6)
[0042] Compound 4 (1g, 7.25mmol), Compound 5 (7.25mmol), X-Phos (0.73mmol), Pd 2 (dba) 3 (0.15mmol), K 3 PO 4 (14.5mmol) and 30ml of ethylene glycol dimethyl ether were added to a 100ml round bottom flask. Under the protection of nitrogen, the reaction was refluxed for 24 h, the solvent was evaporated to dryness under reduced pressure, and the yellow oily compound 6 was obtained by column chromatography (yield 36.26%). 1 H NMR (600MHz, CDCl 3 )δ8.27(d,J=3.0Hz,1H),7.47(d,J=8.8Hz,1H),7.06(dd,J=8.8,3.0Hz,1H),3.40–3.32(m,4H), 2.59–2.50(m,4H),2.32(s,3H).ESI-MS: m / z 203.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com