Preparation method of nicotinamide mononucleotide
A technology of nicotinamide riboside salt and mononucleotide, which is applied in the field of preparation of nicotinamide mononucleotide, and can solve the problem of low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
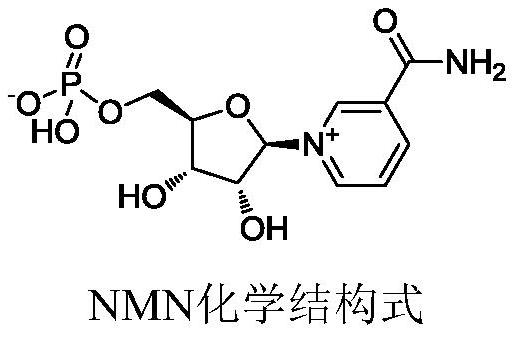
[0027] In a typical embodiment of the present application, a method for preparing nicotinamide mononucleotide is provided, the preparation method comprising: step S1, comprising nicotinamide riboside salt, 1,8-bisdimethylamino The raw materials of naphthalene, phosphorus oxychloride and solvent are phosphorylated to obtain a post-reaction system; in step S2, the post-reaction system is hydrolyzed to obtain a reaction solution containing nicotinamide mononucleotide.
[0028] The 2-hydroxyl on the five-membered heterocyclic ring of nicotinamide riboside salt is the primary hydroxyl group, and the 3 and 4-position hydroxyl groups are secondary hydroxyl groups. Since the primary hydroxyl group is more reactive than the secondary hydroxyl group, when the above phosphorylation reaction occurs, 1, 8-bisdimethylaminonaphthalene preferentially forms a complex with the 2-hydroxyl group of nicotinamide riboside salt, and on the one hand, because 1,8-bisdimethylaminonaphthalene is a rigid ...
Embodiment 1
[0051] Weigh 10g nicotinamide ribose chloride (34.4mmol), add it to a 100mL reaction bottle, add 50mL trimethyl phosphate, cool down to -20~-10°C, add 11.06g 1,8-bisdimethylaminonaphthalene (51.6mmol) , and then dropwise added 26.4g of phosphorus oxychloride (172mmol), reacted for 24h, filtered the reaction solution under reduced pressure and concentrated the filtrate under reduced pressure to obtain a concentrated product.
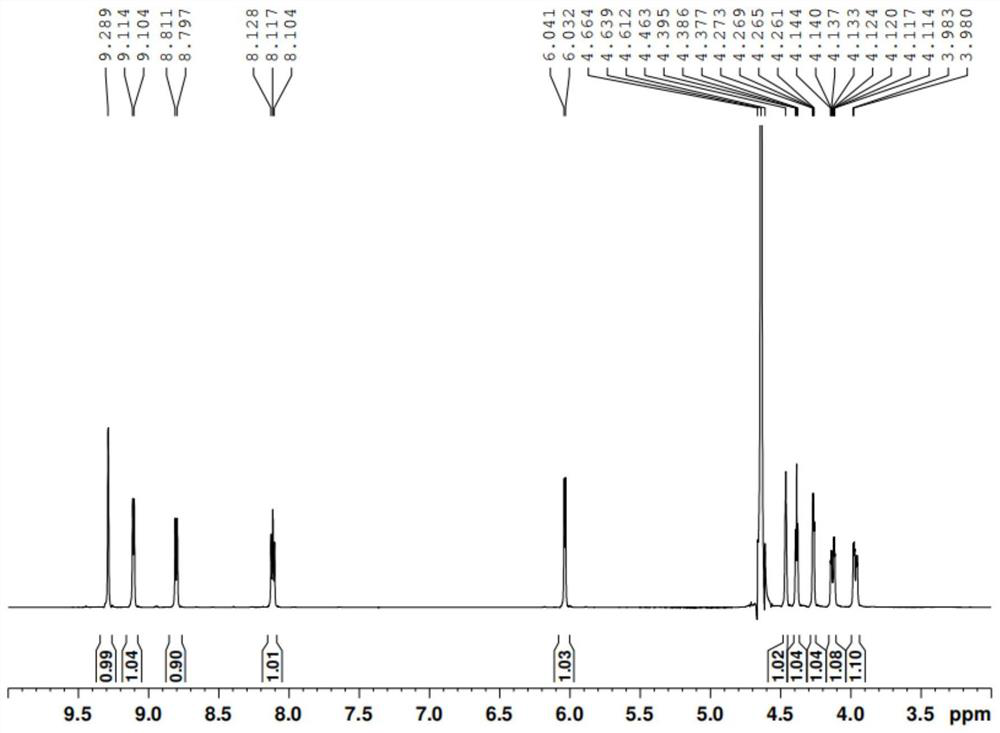
[0052] Add 20mL of purified water to the concentrated product at a temperature of -10~-5°C. After dissolving, slowly add 200mL of acetonitrile dropwise, stir and crystallize for 4 hours, and filter to obtain 8.62g of β-nicotinamide mononucleotide. The proton nuclear magnetic resonance spectrum of β-nicotinamide mononucleotide products is attached figure 1 , and the H-NMR spectrum data are: 1 H-NMR (600MHz,D 2 ( 1H,d,H-1),4.405-4.420(1H,m,H-2),4.388-4.396(1H,t,H-3),4.249(1H,d,H-4),4.045-4.046( 1H,d,H-5), 3.876-3.896(1H,d,H-5). From attached figure 1 I...
Embodiment 2
[0054] The difference between embodiment 2 and embodiment 1 is that
[0055] The molar ratio of 1,8-bisdimethylaminonaphthalene, nicotinamide ribose chloride (34.4mmol) and phosphorus oxychloride was 1:1:5, and finally 8.05g of β-nicotinamide mononucleotide was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com