Antioxidant composition containing NMN and preparation method thereof
A composition and anti-oxidation technology, which is applied in the directions of medical preparations, drug combinations, and antidote containing active ingredients to achieve the effects of stable structure, proper compatibility, and not easy to deteriorate and damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
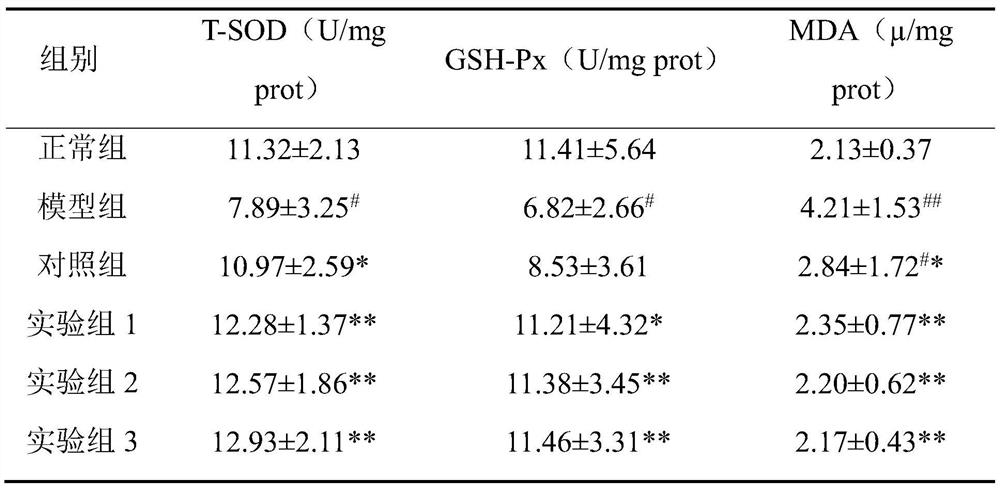
Examples
Embodiment 1
[0046] The preparation of embodiment 1 composition capsule
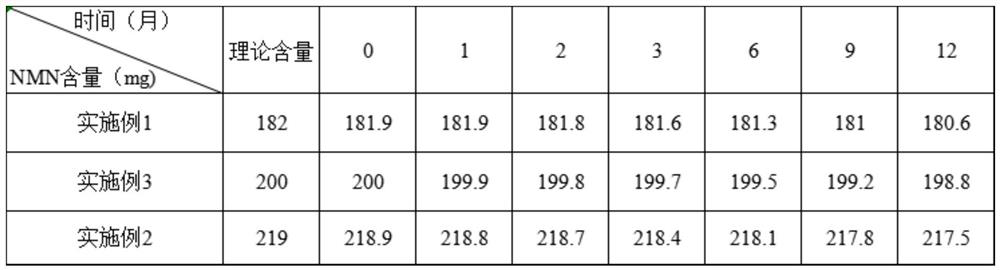
[0047] (1) Active raw materials: 10.64g NMN, 0.6g coenzyme Q10 aqueous solution (coenzyme Q10 mass percentage content: 10%), 0.45g plasmalogen, 0.96g DNA, 0.96g RNA, 0.28g L-tryptophan, 0.2g L-tyrosine, 0.06g riboflavin (VB2), 0.06g niacin, 0.04g pyridoxine hydrochloride (VB6), 0.06g vitamin C (L-ascorbic acid P), 0.06g dry vitamin E, 0.15g containing Zinc yeast (10% by mass percentage of zinc), 0.15g of yeast containing manganese (5% by mass percentage of manganese), 0.15g of yeast containing selenium (0.2% by mass percentage of selenium).
[0048] (2) Excipients: 0.38g calcium stearate
[0049] (3) Preparation method:
[0050] The dried raw materials and auxiliary materials are respectively passed through a 60-mesh sieve, and then the screened raw materials are weighed in proportion, and relevant auxiliary materials are added at the same time, and mixed evenly. After granulation, drying, granulation and other pr...
Embodiment 2
[0052] The preparation of embodiment 2 composition capsules
[0053] (1) Active raw materials: 12g NMN, 0.48g coenzyme Q10 aqueous solution (coenzyme Q10 mass percentage content: 10%), 0.24g plasmalogen, 0.96g DNA, 0.96g RNA, 0.08g L-tryptophan, 0.06g L- Tyrosine, 0.04g riboflavin (VB2), 0.06g niacin, 0.04g pyridoxine hydrochloride (VB6), 0.06g vitamin C (L-ascorbic acid P), 0.06g dry vitamin E, 0.06g zinc yeast (10% by mass percentage of zinc), 0.06g of yeast containing manganese (5% by mass percentage of manganese), and 0.06g of yeast containing selenium (0.2% by mass percentage of selenium).
[0054] (2) Excipients: 0.38g calcium stearate
[0055] (3) Preparation method:
[0056] The dried raw materials and auxiliary materials are respectively passed through a 60-mesh sieve, and then the screened raw materials are weighed in proportion, and relevant auxiliary materials are added at the same time, and mixed evenly. After granulation, drying, granulation and other processe...
Embodiment 3
[0058] The preparation of embodiment 3 composition capsules
[0059] (1) Active raw materials: 12.8g NMN, 0.28g coenzyme Q10 aqueous solution (coenzyme Q10 mass percentage content: 10%), 0.26g plasmalogen, 0.46g DNA, 0.46g RNA, 0.04g L-tryptophan, 0.04g L - Tyrosine, 0.06g riboflavin (VB2), 0.08g niacin, 0.04g pyridoxine hydrochloride (VB6), 0.06g vitamin C (L-ascorbic acid P), 0.06g dry vitamin E, 0.06g zinc Yeast (10% by mass percentage of zinc), 0.06g of yeast containing manganese (5% by mass percentage of manganese), 0.06g of yeast containing selenium (0.2% by mass percentage of selenium).
[0060] (2) Excipients: 0.38g calcium stearate
[0061] (3) Preparation method:
[0062] The dried raw materials and auxiliary materials are respectively passed through a 60-mesh sieve, and then the screened raw materials are weighed in proportion, and relevant auxiliary materials are added at the same time, and mixed evenly. After granulation, drying, granulation and other processes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com