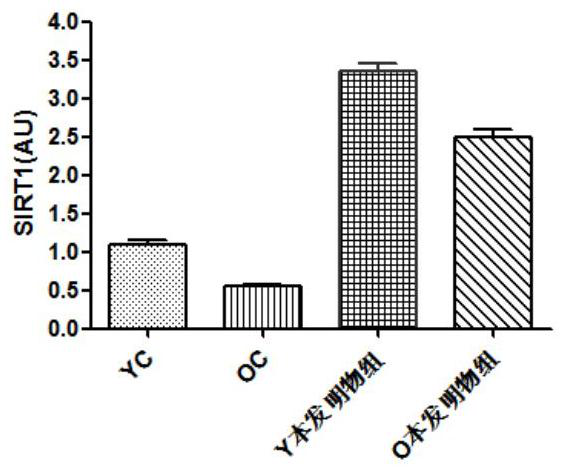
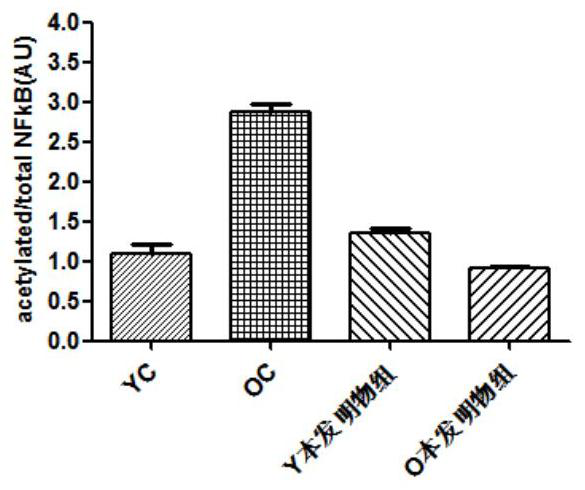
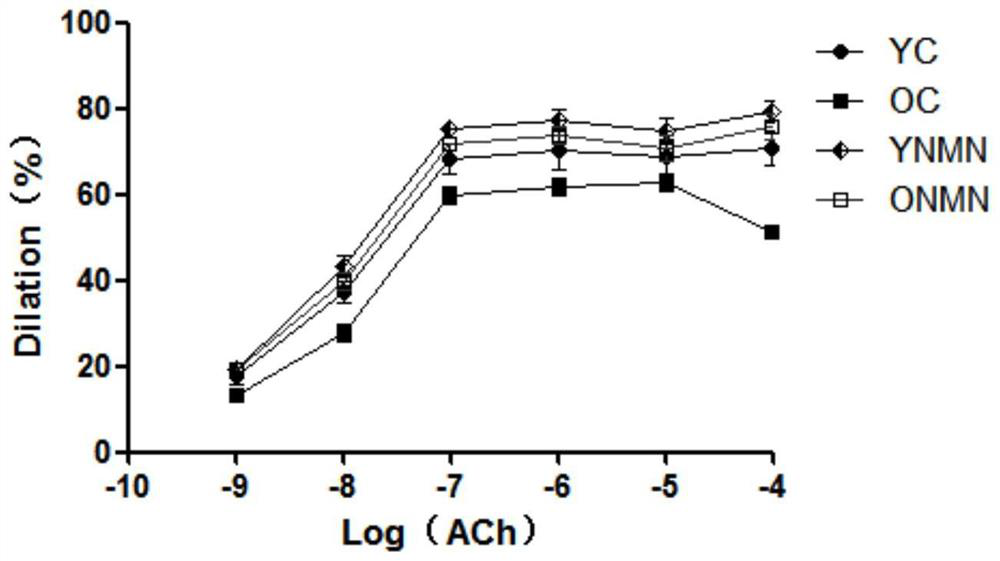
Application of the composition containing nicotinamide mononucleotide in anti-aging medicine/health product
A single nucleotide and composition technology, which is applied in the direction of medical preparations, applications, and drug combinations containing active ingredients, can solve problems such as difficult to take into account the anti-aging effect and product quality stability, and achieve good anti-aging effect and stable structure , the effect of not easy to deteriorate and damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This embodiment is a specific embodiment for preparing product 1.
[0043] Niacinamide mononucleotide, evodiamine, resveratrol, sumac, butaein, icariin and honokiol were dried separately, passed through a 60-80 mesh sieve, and mixed uniformly to obtain the first product;
[0044] After the auxiliary materials are dried, mix them evenly, and pass through a 60-80 mesh sieve to obtain the second product; in this embodiment, the auxiliary materials are mannitol.
[0045] After the first product and the second product are dissolved in water, stir for the first time at 25-40°C, heat up after stirring evenly, then stir for the second time at 50°C, and after stirring for 1 hour, the preparation is tablet product 1; In the examples, the formulation process used is a conventional formulation process well known to those skilled in the art, and will not be repeated here.
[0046] In this example, in the first product, the dosage of each raw material is: 10g of nicotinamide mononuc...
Embodiment 2
[0048] This embodiment is a specific embodiment for preparing product 2.
[0049] Niacinamide mononucleotide, evodiamine, resveratrol, sumac, butaein, icariin and honokiol were dried separately, passed through a 60-80 mesh sieve, and mixed uniformly to obtain the first product;
[0050] After the auxiliary materials are dried, mix them evenly, and pass through a 60-80 mesh sieve to obtain the second product; in this embodiment, the auxiliary materials are microcrystalline cellulose.
[0051] After the first product and the second product are dissolved in water, stir for the first time at 25-40°C, heat up after stirring evenly, then stir for the second time at 50°C, and after stirring for 1 hour, the product 2 is obtained as a pill; this implementation In the example, the formulation process used is a conventional formulation process well known to those skilled in the art, and will not be repeated here.
[0052] In this example, in the first product, the dosage of each raw mat...
Embodiment 3
[0054] This embodiment is a specific embodiment for preparing product 3.
[0055] Niacinamide mononucleotide, evodiamine, resveratrol, sumac, butaein, icariin and honokiol were dried separately, passed through a 60-80 mesh sieve, and mixed uniformly to obtain the first product;
[0056] After the auxiliary materials are dried, mix them evenly, and pass through a 60-80 mesh sieve to obtain the second product; in this embodiment, the auxiliary materials are magnesium stearate.
[0057] After the first product and the second product are dissolved in water, stir for the first time at 25-40°C, heat up after stirring evenly, then stir for the second time at 50°C, and after stirring for 1 hour, the preparation is granule product 3; In the examples, the formulation process used is a conventional formulation process well known to those skilled in the art, and will not be repeated here.
[0058] In this example, in the first product, the dosage of each raw material is: 2 g of nicotinam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com