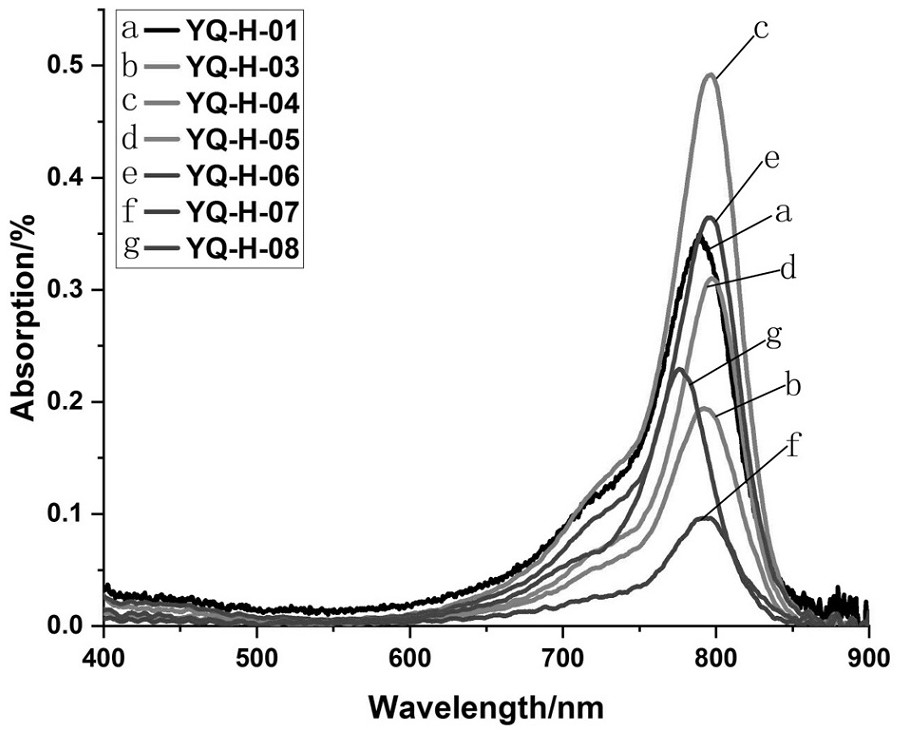
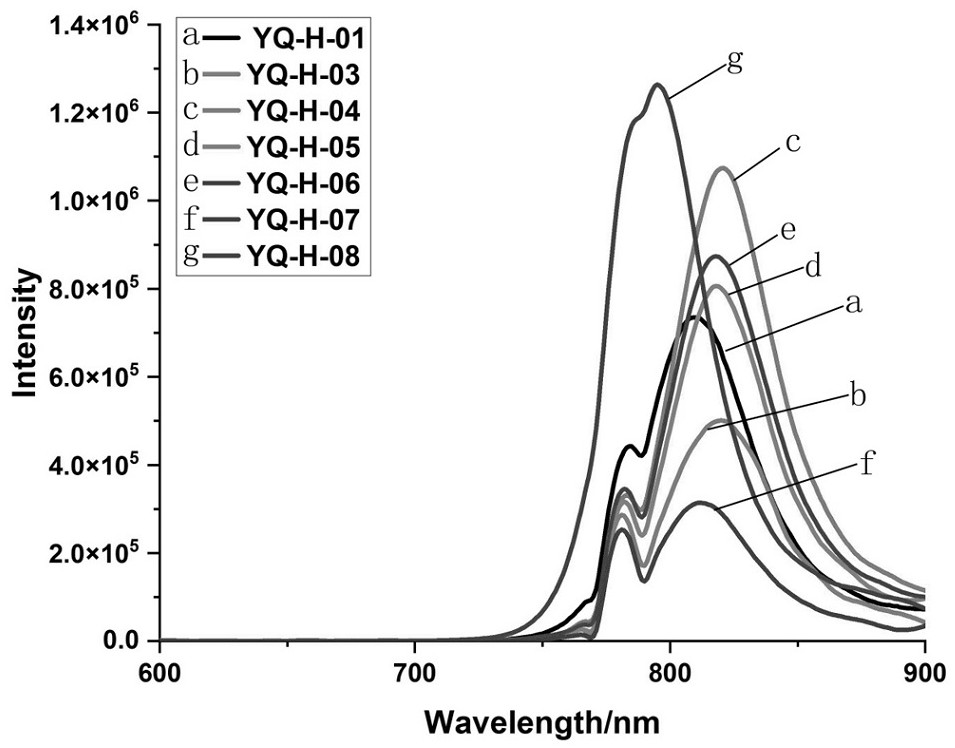
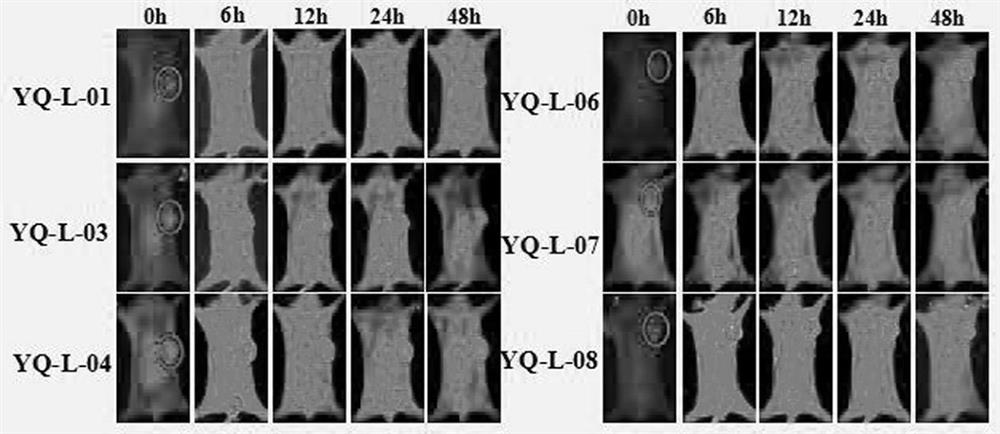
Near-infrared fluorescent probe for specifically targeting tumor as well as synthesis method and application of near-infrared fluorescent probe
A technology of fluorescent probe and synthesis method, applied in the field of specific molecular targeted diagnostic reagents, which can solve the problems of false positives of ICG, unfavorable imaging of liver and intestinal tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the synthesis of YQ-H-01
[0034]
[0035]
[0036] Lapatinib (5mg, 1.0eq), ICG-02 (12mg, 1.5eq), 2-(7-azabenzotriazole)-N,N,N',N'-tetramethylurea Fluorophosphate (HATU, 13mg, 4.0eq), triethylamine (6ul, 5.0eq), reacted at room temperature in the dark for 3 hours, and monitored the reaction by HPLC. After the reaction was complete, it was purified by preparative liquid phase, and the target fraction was freeze-dried to obtain Green solid YQ-H-01 (5 mg, Y=38.9%), confirmed by mass spectrometry and NMR spectroscopy, m / z=, 1 H NMR (300 MHz, DMSO) δ 8.88 (dd, J = 15.1,10.5 Hz, 2H), 8.66 (t, J = 12.4 Hz, 2H), 8.24 (dd, J = 11.1, 6.4 Hz, 1H), 8.01 (t, J = 2.8 Hz, 1H), 7.85-7.66 (m, 4H), 7.60-7.45 (m, 3H), 7.42-7.16 (m,7H), 6.52 (dt, J = 24.9, 7.5 Hz, 3H), 5.34 (s, 2H), 4.64 (s, 2H), 4.26 (d, J = 7.2 Hz, 4H), 3.39 (t, J = 8.0 Hz, 2H), 3.17 (s, 2H), 3.04 (d, J = 9.4 Hz,4H), 2.73-2.59 (m, 8H), 2.55 (s, 3H), 2.07-1.88 (m, 4H), 1.78-1.65 (m, 2H),...
Embodiment 2
[0037] Embodiment 2: the synthesis of YQ-H-03
[0038]
[0039]
[0040] Lapatinib (10mg, 1.0eq), 6-Boc-aminocaproic acid (8 mg, 2eq), 2-(7-azabenzotriazole)-N,N,N',N'-tetra Methylurea hexafluorophosphate (HATU, 13mg, 2.0eq), triethylamine (7.2ul, 3.0eq), react at room temperature for 1 hour, TLC monitors the reaction, after the reaction is complete, drop the reaction solution into water, and then Ethyl acetate was extracted, and the combined organic layer was concentrated and added trifluoroacetic acid to remove Boc. The reaction was also monitored by TLC. After the reaction was complete, the trifluoroacetic acid was evaporated and purified to obtain lapatinib linked to 6-aminocaproic acid. Subsequent synthesis of the reference compound YQ-H-01 yielded the target compound YQ-H-03, a green solid, whose structure was confirmed by mass spectrometry and nuclear magnetic proton spectroscopy, m / z=, 1H NMR (300 MHz, DMSO) δ 8.88 (dd, J = 15.1,10.5 Hz, 2H), 8.66 (t, J = 12.4 H...
Embodiment 3
[0041] Embodiment 3: the synthesis of YQ-H-04
[0042]
[0043] The synthesis method refers to YQ-H-03, the structure is confirmed by mass spectrometry and NMR spectrum, m / z=, 1 H NMR (300 MHz, DMSO) δ 8.96 (dd, J = 8.0, 4.8 Hz, 2H), 8.66 (d, J = 14.0 Hz, 2H), 8.36 (d, J = 8.9 Hz, 1H), 8.01 -7.88 (m, 3H), 7.75 (d, J = 1.2 Hz, 2H), 7.65 (ddd, J =9.6, 8.6, 1.9 Hz, 3H), 7.53-7.46 (m, 1H), 7.45-7.30 (m, 5H ), 7.25-7.14 (m,2H), 6.64-6.43 (m, 3H), 5.32 (s, 2H), 4.72 (d, J = 23.4 Hz, 2H), 4.40-4.23(m, 4H), 3.61- 3.53 (m, 2H), 3.51-3.41 (m, 12H), 3.41-3.30 (m, 4H), 3.19-3.12(m, 2H), 3.03 (d, J = 4.6 Hz, 2H), 2.97 (td, J = 6.6, 3.9 Hz, 2H), 2.76 (dd, J = 11.9, 5.0 Hz, 2H), 2.73-2.59 (m, 8H), 2.55 (s, 3H), 2.42-2.31 (m, 2H), 2.10 -1.91 (m, 4H), 1.80-1.71 (m, 2H), 1.65 (d, J = 2.0 Hz, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com