Synthesis method of (-)-huperzine A
A synthesis method and technology of huperzine A, applied in the field of drug synthesis, can solve problems such as many types of impurities, and achieve the effects of less types of impurities, high chemical and optical purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
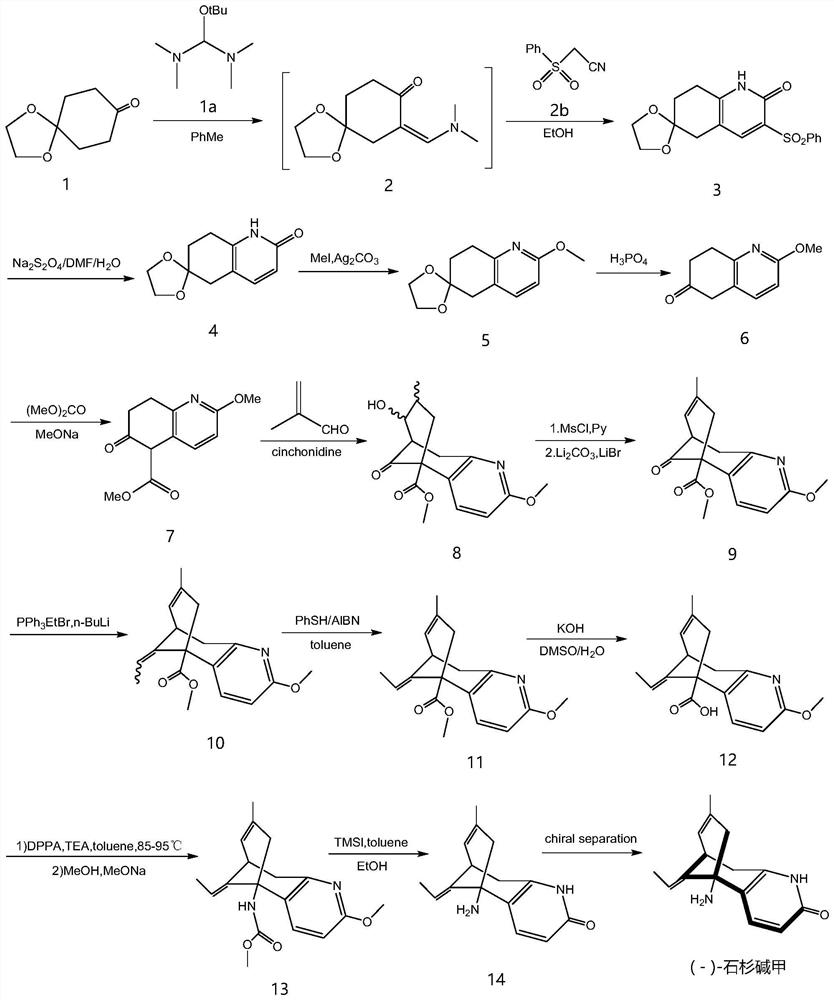
[0060] The present embodiment provides a kind of synthetic method of (-)-huperzine A, and its synthetic route is as follows figure 1 Shown:
[0061] Each reaction step is as follows:
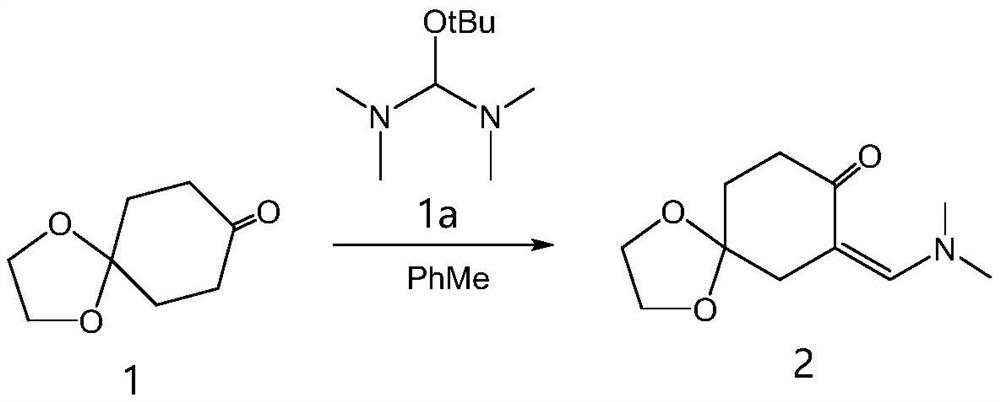
[0062] Synthesis of step 1, compound 2
[0063] The reaction formula is as follows:
[0064]
[0065] The specific preparation process is: in a three-necked flask, first add toluene (1L), then add 1,4-cyclohexanedione monoethylene glycol ketal (compound 1, 1.6mol) and compound 1a (3mol), and heat up to The reaction was stirred at 75°C, and the progress of the reaction was detected by TLC. After 2 hours, the reaction was complete, and the heating was stopped. The reaction solution was directly spin-dried to obtain compound 2 (yield 80%). MS, 'HNMR are consistent with the structure.
[0066] Step 2, the synthesis of compound 3
[0067] The reaction formula is as follows:
[0068]
[0069] The specific preparation process is as follows: in a 2L three-necked flask, respectively add comp...
Embodiment 2
[0121] Each step is with embodiment 1, and difference is:
[0122] In step 1, the molar ratio of compound 1 and compound 1a is 1:1.5, and the reaction temperature is 70°C.
[0123] In step 6, compound 6, MeONa and (MeO) 2 The molar ratio of CO was 1:1:1.5, the reaction temperature was 85°C, and the reaction time was 5 hours.
[0124] In step 7, the molar ratio of compound 7, cinchonidine and methacrolein was 1:1:2, and the reaction was carried out at room temperature for 5 hours.
[0125] In step 8, compound 8, MsCl, Py, Li 2 CO 3 The molar ratio to LiBr is 1:1:0.8:0.5:0.5, the reaction is carried out at a temperature of 100° C. for 3 hours, and the stirring time is 20 minutes.
[0126] In step 9, compound 9 and PPh 3 The molar ratio of EtBr was 1:1.8, and the reaction was carried out at a temperature of 20° C. for 3 hours.
[0127] In step 11, the solvent is an aqueous solution of DSMO, the base is an aqueous KOH solution with a mass fraction of 20%, the volume ratio of...
Embodiment 3
[0135] Each step is with embodiment 1, and difference is:
[0136] In step 1, the molar ratio of compound 1 and compound 1a is 1:3, and the reaction temperature is 80°C.
[0137] In step 6, compound 6, MeONa and (MeO) 2 The molar ratio of CO was 1:1.5:2.5, the reaction temperature was 90°C, and the reaction time was 8 hours.
[0138] In step 7, the molar ratio of compound 7, cinchonidine and methacrolein was 1:1.5:3, and the reaction was carried out at room temperature for 8 hours.
[0139] In step 8, compound 8, MsCl, Py, Li 2 CO 3 The molar ratio to LiBr is 1:1.5:1.2:1.5:1.5, the reaction is carried out at a temperature of 120° C. for 5 hours, and the stirring time is 30 minutes.
[0140] In step 9, compound 9 and PPh 3 The molar ratio of EtBr was 1:2.4, and the reaction was carried out at a temperature of 35° C. for 8 hours.
[0141] In step 11, the solvent is an aqueous solution of DSMO, the base is an aqueous KOH solution with a mass fraction of 30%, the volume rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com