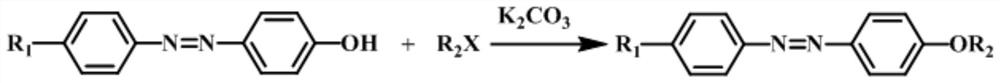Etherification method of para-alkane azophenol and halogenated alkane
A technology for alkane-based azophenol and halogenated alkane, which is applied in the field of etherification of para-alkane-based azophenol, can solve the problems of low conversion rate and the like, and achieves the effects of mild reaction conditions and improved reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
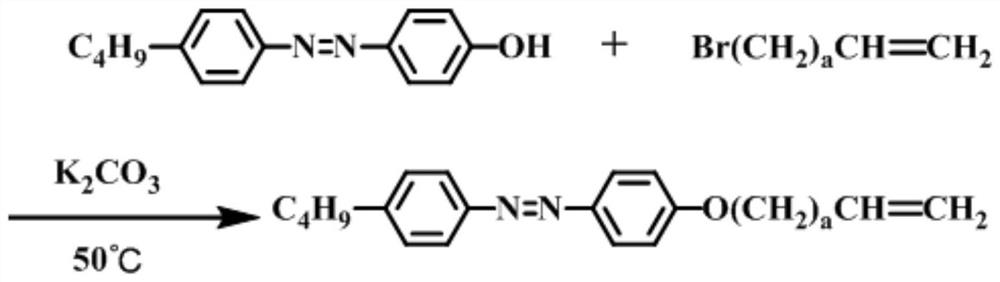
[0024] DETAILED DESCRIPTION OF THE INVENTION An etherification method of alkane the alkane-based coupling phenol and a halogenated alkane is performed as follows:
[0025] First, alkalization treatment:
[0026] The counterkylene alkane group was dissolved in acetone, and then an alkalization agent was added, and the alkalization treatment was administered for 30 min to 60 min to obtain an alkalized mixture.
[0027] The molar ratio of the opposite alkane based azo phenol and an alkalizing agent is 1: (3 ~ 5);
[0028] Second, the etherification reaction:
[0029] The haloalkane is added to the alkalized mixture, and the reflux reaction is refluxed for 12 h to 48 h under a nitrogen atmosphere and stirring conditions at a nitrogen atmosphere and stirring conditions.
[0030] The molar ratio of the opposite alkane group and the halogenated alkane is 1: (1 ~ 3);
[0031] Third, the removal of the alkalization agent:
[0032] Dilute hydrochloric acid is added to the solution after the...
specific Embodiment approach 2
[0040] DETAILED DESCRIPTION OF THE INVENTION The present embodiment is different from that of the particular embodiment: the counter-alkane-based coupling phenol described in the step one is Rim 1 Other alkane groups of different carbon chain lengths are identical to those of the specific embodiments.
specific Embodiment approach 3
[0041] DETAILED DESCRIPTION OF THE INVENTION 3: The present embodiment is different from one or two of the specific embodiments that the alkalization agent described in steps is sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate. Others are the same as those of the specific embodiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com