Preparation method of 4-methoxy-2-nitroaniline
A technology of methoxyaniline and nitroaniline, which is applied in the field of preparation of 4-methoxy-2-nitroaniline, can solve the problems of long reaction route, low process yield, harsh reaction conditions, etc. Reduced risk factor, simple reaction operation, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A preparation method of 4-methoxy-2-nitroaniline, the synthetic route is as follows:
[0029] Concrete synthetic steps include:
[0030] 1) Compound (1) N -Benzenesulfonyl-4-methoxyaniline is dissolved in 1,2-dichloroethane, added in pyridine and copper nitrate trihydrate, and reacted when the solution temperature reaches 90°C-115°C N -Benzenesulfonyl-4-methoxy-2-nitroaniline; after the reaction is completed, pour the reaction solution into ice water, adjust the pH = 6, extract 3 times with 1,2-dichloroethane, combine the organic phases, Dry over anhydrous sodium sulfate, filter, wash the filter cake with 1,2-dichloroethane, and remove the solvent under reduced pressure to obtain (2) N - Crude benzenesulfonyl-4-methoxy-2-nitroaniline. Among them, compound (1) N -Benzenesulfonyl-4-methoxyaniline and copper nitrate trihydrate react with a substance ratio of 1: (1-5).
[0031] 2) Compound (2) N Add the crude product of -benzenesulfonyl-4-methoxy-2-nitroaniline to 1,2...
Embodiment 1
[0035] First Synthesis of Compounds (2) N -Benzenesulfonyl-4-methoxy-2-nitroaniline
[0036] Compound (1) N -Benzenesulfonyl-4-methoxyaniline (0.2 mol), pyridine (0.3 mol), and copper nitrate trihydrate (0.3 mol) were added to 200 mL of 1,2-dichloroethane, and after the raw materials were dissolved, Heat the reaction system to 95°C-105°C, generally 100°C, monitor the reaction process with LCMS and HPLC, and process it when the reaction time reaches 12 h. The reaction solution was poured into 1 times the volume of ice water, and the pH was adjusted to pH = 6. A large amount of solids precipitated, extracted three times with 100 mL of 1,2-dichloroethane each time, combined the organic phases, dried over anhydrous sodium sulfate, and filtered. 1,2-Dichloroethane to wash the filter cake. The filtrate was removed in a vacuum to obtain 47.7 g of compound (2) N -Benzenesulfonyl-4-methoxy-2-nitroaniline, yield 74%, HPLC purity 95%.
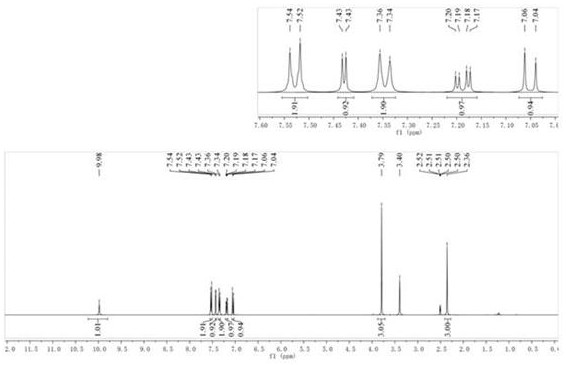
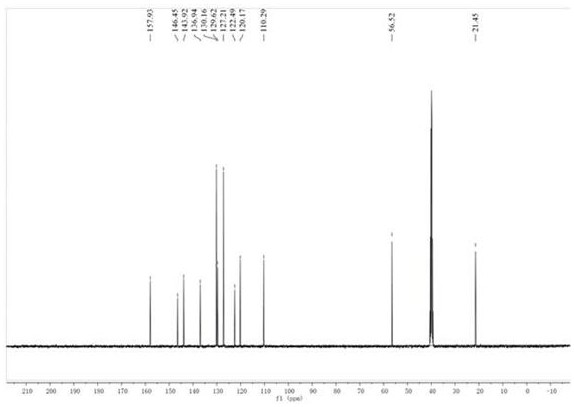
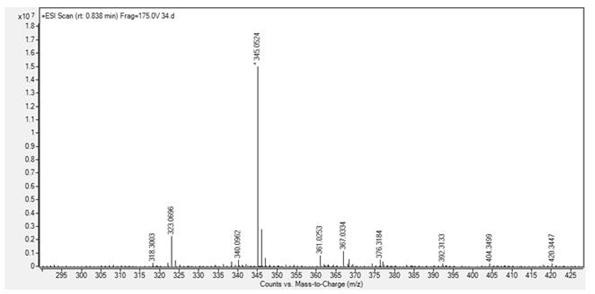
[0037] compound (2) N The H NMR spectrum of -...
example 2
[0046] First Synthesis of Compounds (2) N -Benzenesulfonyl-4-methoxy-2-nitroaniline
[0047] Compound (1) N -Benzenesulfonyl-4-methoxyaniline (0.2 mol), pyridine (0.3 mol), and copper nitrate trihydrate (0.2 mol) were added to 200 mL of 1,2-dichloroethane, and after the raw materials were dissolved, Heat the reaction system to 95°C-105°C, generally 100°C, monitor the reaction process with LCMS and HPLC, and process it when the reaction time reaches 12 h. Pour the reaction liquid into 1 times the volume of ice water, adjust the pH to PH = 6, a large amount of solids precipitated, extracted three times with 100 mL 1,2-dichloroethane each time, combined the organic phases, dried over anhydrous sodium sulfate, filtered, 1,2-dichloroethane to wash the filter cake. The filtrate was vacuum removed to obtain 41 g of compound (2) N -Benzenesulfonyl-4-methoxy-2-nitroaniline, yield 63%, HPLC purity 94%.
[0048] The second step to synthesize compound (3) 4-methoxy-2-nitroaniline
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com