Preparation method of myricetin-loaded nano-micelle for reducing ovariectomy-induced bone loss by inhibiting formation of osteoclast
A technology for inhibiting osteoclastosis and ovariectomy, applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problems of low release and bioavailability in vitro, and achieve good bone targeting in vivo and in vitro , good therapeutic effect, and the effect of improving ALP activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of bone targeting material AL-P(LLA-CL)-PEG-P(LLA-CL):
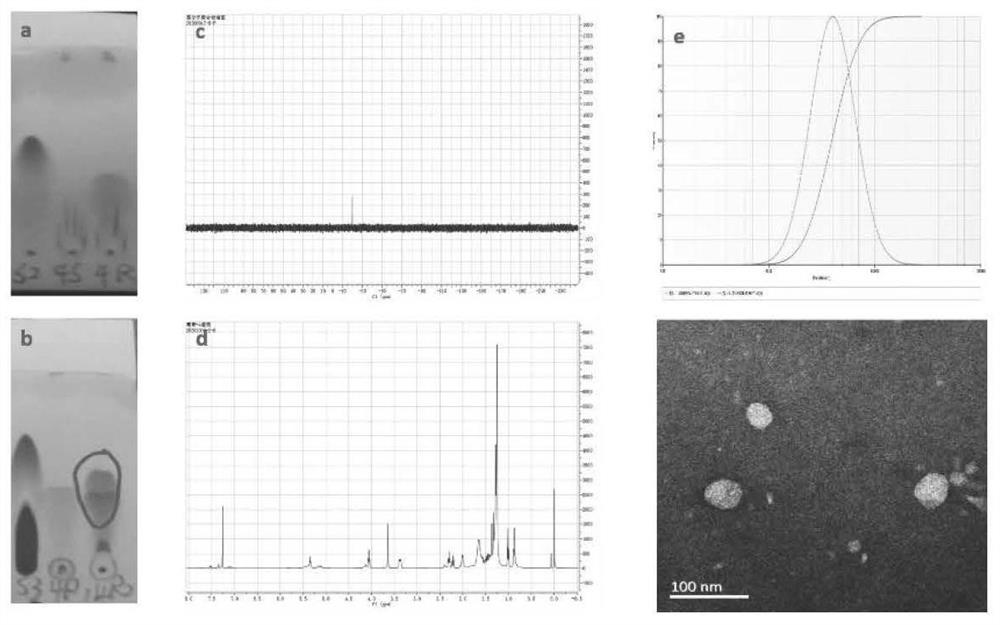
[0031]The compound P(LLA-CL)5 / 5(4500)-PEG(2000)-P(LLA-CL)5 / 5(4500) was ultrasonically dissolved in the DMF solution, and the compound CDI(N,N' was added while stirring -carbonyldiimidazole), reacted at room temperature for 5h. Use dichloromethane-methanol (15:1, v / v) as developing solvent to detect related substances. After the first step reaction finishes, add the mixed solution of alendronate sodium (AL) and tetrabutylammonium hydroxide (molar ratio=1:2; Alendronate sodium, the amount of trihydrate) while stirring in the reaction liquid 10mg, 0.03mmol; tetrabutylammonium hydroxide aqueous solution is 38.9mg, 0.06mmol), heated at 80°C for 5h. The progress of the reaction was monitored by running a plate with dichloromethane:methanol=15:1. After the reaction, use a rotary evaporator to remove DMF (70-80°C), add a small amount of water to wash the remaining reaction solution, and centrifuge to remove t...
Embodiment 2
[0034] Preparation of myricetin-loaded nanomicelles that reduce ovariectomy-induced bone loss by inhibiting osteoclast formation:
[0035] Dissolve the dried AL-P(LLA-CL)-PEG-P(LLA-CL) in ultrapure water at a concentration of 10 mg / mL. Myricetin (MY) was dissolved in absolute ethanol to prepare a solution with a concentration of 10 mg / mL. Take 1, 2, 3, and 4 mL of the prepared MY, and slowly drop them into 20 mL of 10 mg / mL AL-P(LLA-CL)-PEG-P (LLA-CL) aqueous solution under ultrasonic conditions, and ultrasonically mix Uniform (ultrasonic power 200W, ultrasonic time 20min). Remove absolute ethanol by rotary evaporation under reduced pressure, and then filter with a 0.45 μm water filter to obtain AL-P(LLA-CL)-PEG-P(LLA-CL)-MY drug-loaded micelles. The particle size, potential and polydispersity coefficient of the four groups of micelles were measured respectively, and the four groups of micelles were placed in a room temperature environment for 7 days, and the particle size o...
Embodiment 3
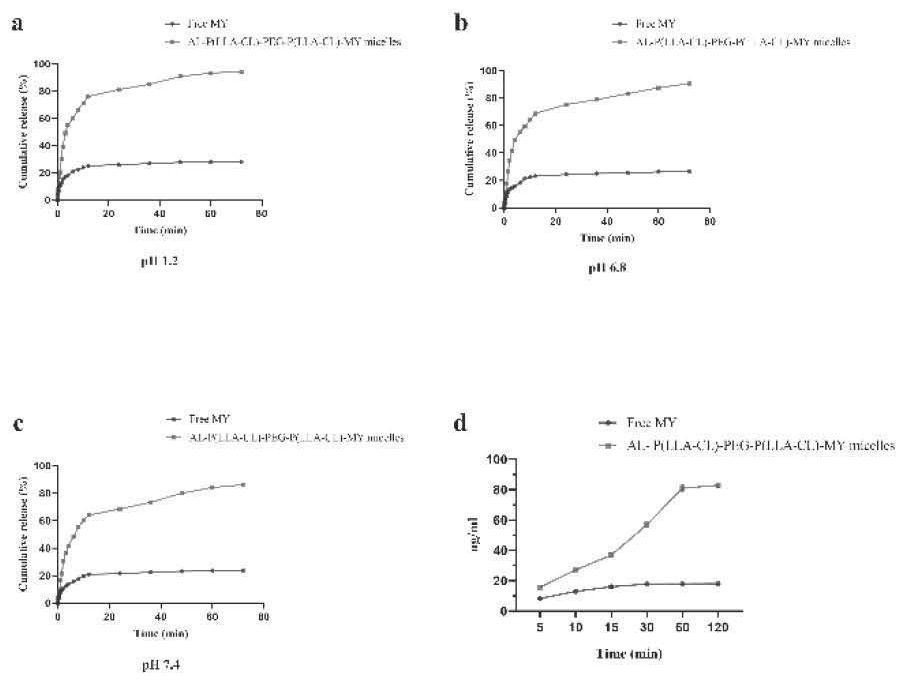
[0041] Targeting study of myricetin micelles
[0042] Add nano-scale hydroxyapatite aqueous solution into two 2mL plastic centrifuge tubes respectively, and then add 500uL of myricetin raw material drug and preparation solution (1mg / mL), sonicate for 5min to mix evenly, let it stand at room temperature, and add it at different times Point 5min, 10min, 15min, 30min, 60min, 120min for testing. Take one tube for centrifugation (10000rpm, 5min), take the supernatant, pass through a 0.22um filter, and perform HPLC detection, repeat three times at each time point, take the average value, and calculate the myricetin content; the same method is used, except that nano-scale Aqueous hydroxyapatite solution was used to test the release of formulations at different time points.
[0043] The results showed that after 1 h, the binding rate of myricetin and hydroxyapatite in myricetin-targeted micelles was higher than 80%, while the binding rate of free myricetin and hydroxyapatite was lowe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com