Anti-MMAE monoclonal antibody as well as preparation method and application thereof
A monoclonal antibody and antibody technology, applied in the field of biomedicine, can solve the problems of difficult MMAE analysis and high requirements for personnel and instruments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Antigen Preparation
[0027] The company synthesizes MMAE small molecules in-house, and labels a cysteine (MMAE-CYS) to facilitate coupling with carrier proteins for immunization or detection. Carrier protein coupling, immune antigen preparation, because MMAE molecules are relatively small and highly toxic, it is not suitable for direct immunization, MMAE-CYS is coupled with carrier protein, choose Thermo Scientific maleamide-activated keyhole blue (KLH) For the protein kit, follow the procedure provided by the kit to perform coupling with the carrier protein.
Embodiment 2
[0028] Example 2: Animal immunization
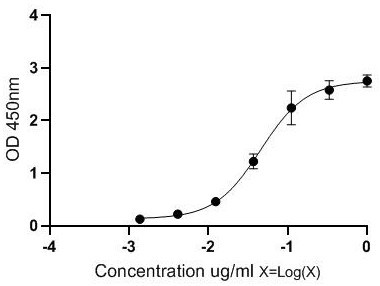
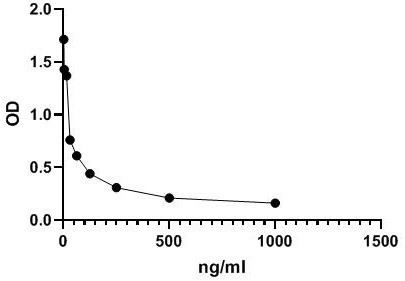
[0029] The antigen for immunization (MMAE-CYS-KLH) prepared in Example 1 was emulsified with complete Freund's adjuvant, and immunized with 4-6 week-old female Balb / c mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. ), subcutaneously injected into the abdomen, 6 points per mouse, and the dose was 100 μg / only. Immunization was boosted once every 14 days, and the antigen was emulsified with Freund's incomplete adjuvant at a dose of 50 µg per mouse. Seven days after the third booster immunization, the polyantibody titer against the immunogen in the mouse serum was detected by indirect ELISA (wavelength 450nm). The dose is 50µg / only.
Embodiment 3
[0030] Embodiment 3: hybridoma cell preparation
[0031] 1. Fusion: Blood was collected from the mouse that reached the immune standard in Example 2, and the spleen was taken to prepare a cell suspension, which was mixed with mouse myeloma cells F0 at a ratio of 10:1, and centrifuged at 1200 rpm for 5 minutes. After discarding the supernatant, put the centrifuge tube into a 37°C water bath, slowly add 1 mL of PEG1500 (Roche Company) within 1 minute, and mix the cells evenly. After standing in warm water for 1 minute, add 10 mL of serum-free IMDM (Hyclone Company), mix well, and centrifuge at 1000 rpm for 5 minutes. After the supernatant was discarded, 10 mL of IMDM medium containing 10% serum (Excell Company) was added, the cells were gently blown up, and the prepared HAT complete medium (Sigma Company) was added to mix well, and the 96-well plate was spread. Put the culture plate with cells in 37°C, 5% CO 2 cultured in an incubator.
[0032] 2. Screening and subcloning: EL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com