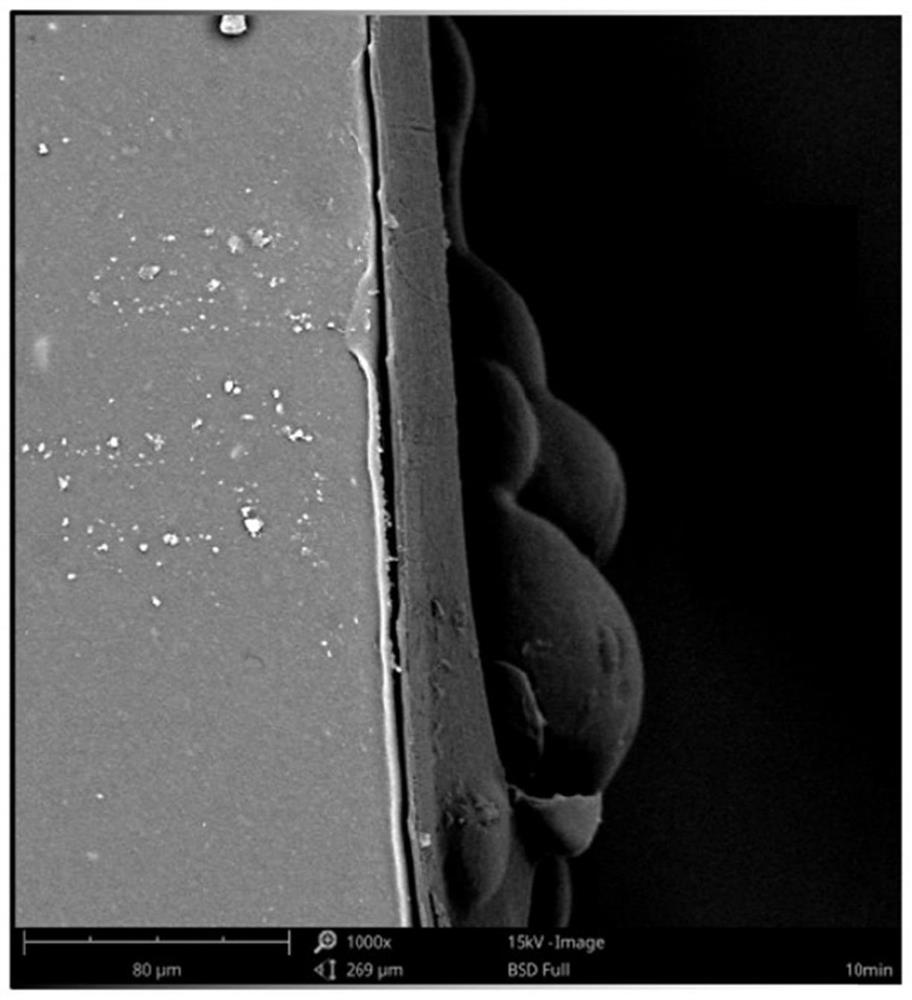
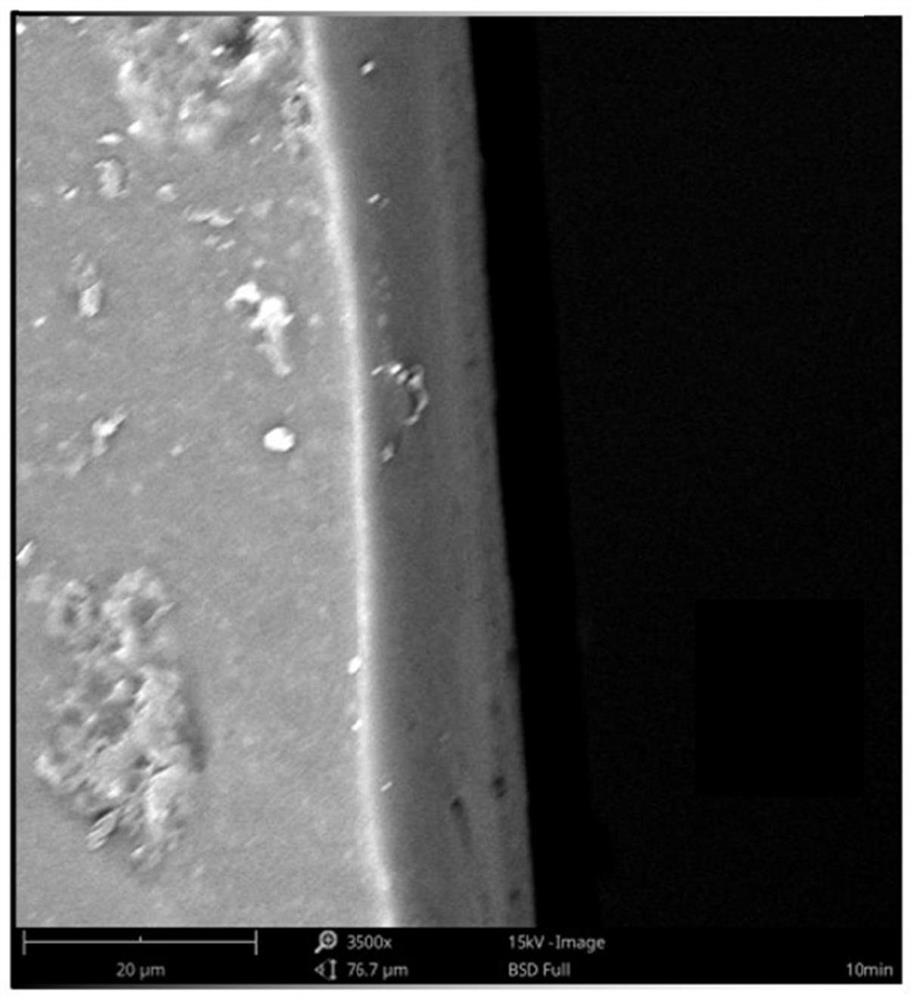
Antibacterial coating as well as preparation method and application thereof
An antibacterial coating and coating technology, applied in coatings, pharmaceutical formulations, catheters, etc., can solve the problems of single composition of antibacterial coatings, uncontrolled drug release, bacterial resistance, etc., to achieve excellent antibacterial effect, maintain Effectiveness of bactericidal concentration time, excellent lubricating action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment has prepared a kind of silicone rubber that is coated with drug-loaded microsphere coating, and specific process is:
[0047]S1: Preparation of CHI-C solution: Chitosan (130 kDa, 3.25 mmol) and dihydroxyphenylpropionic acid (HCA, 6.49 mmol) were dissolved in PBS (50 mL, pH=5). Then EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 6.49mmol) was added to the mixture, stirred at room temperature for 12 hours, after extensive dialysis (Mw: 3500) Afterwards, store the product in a centrifuge tube.
[0048] S2: Preparation of antibacterial coating: Take 1.8g of CHI-C solution, add 20mg of triclosan-loaded PLGA microspheres and 20mg of chlorhexidine-loaded PLGA microspheres, and stir on a magnetic stirrer at a stirring rate of 500rpm-1000rpm ;
[0049] S3: Preparation of silicone rubber: Spin-coat the mixed solution on the surface of the silicone rubber, and dry in an oven at 50°C.
Embodiment 2
[0051] This embodiment has prepared a kind of silicone rubber that is coated with drug-loaded microsphere coating, and specific process is:
[0052] S1: Preparation of CHI-C solution: Chitosan (130 kDa, 3.25 mmol) and dihydroxyphenylpropionic acid (HCA, 3.25 mmol) were dissolved in PBS (50 mL, pH=5). Then EDC (3.25 mmol) was added to the mixture, stirred at room temperature for 12 hours, and after extensive dialysis (Mw: 3500), the product was stored in a centrifuge tube.
[0053] S2: Preparation of antibacterial coating: take 1.8 g of CHI-C solution, add 40 mg of triclosan-loaded PLGA microspheres, and stir on a magnetic stirrer at a stirring rate of 500 rpm to 1000 rpm;
[0054] S3: The mixed solution was spin-coated on the surface of the silicone rubber, and dried in an oven at 50° C. to obtain the silicone rubber CHI-C+TMS.
Embodiment 3
[0056] This embodiment has prepared a kind of silicone rubber that is coated with drug-loaded microsphere coating, and specific process is:
[0057] S1: Preparation of CHI-C solution: Chitosan (130 kDa, 3.25 mmol) and dihydroxyphenylpropionic acid (HCA, 9.75 mmol) were dissolved in PBS (50 mL, pH=5). Then EDC (9.75 mmol) was added to the mixture, stirred at room temperature for 12 hours, and after extensive dialysis (Mw: 3500), the product was stored in a centrifuge tube.
[0058] S2: Preparation of antibacterial coating: take 1.8g of CHI-C solution, add 40mg of chlorhexidine-loaded PLGA microspheres, stir on a magnetic stirrer, and the stirring rate is 500rpm-1000rpm;
[0059] S3: The mixed solution was spin-coated on the surface of the silicone rubber, and dried in an oven at 50° C. to obtain the silicone rubber CHI-C+CMS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com