Preparation of lactic acid bacteria multi-copper oxidase and application of lactic acid bacteria multi-copper oxidase in biogenic amine degradation
A technology of multi-copper oxidase and biogenic amine, which is applied in the field of bioengineering to achieve the effect of good effect, wide application range and good amine reducing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Degradation of biogenic amine by cell disruption liquid of Lactobacillus sake
[0048]The Lactobacillus sakei preserved in our laboratory was inoculated in liquid MRS medium and cultured overnight at 37°C and 200r / min. Centrifuge the bacterial solution at 4°C and 10000r / min for 7min, collect the bacterial sediment, wash the bacterial cells with 20mmol / L pH 7.0 phosphate buffer solution, and then resuspend the bacterial cells with phosphate buffer saline. Add lysozyme at a final concentration of 0.5 mg / ml, place on ice for 30 min, and then freeze and thaw repeatedly 3-5 times in liquid nitrogen to lyse it. The lysate was centrifuged at 4°C and 10,000 r / min for 15 minutes to collect the supernatant, and then the collected solution was passed through a 0.45 μm water filter to obtain the desired crude enzyme solution. Using ABTS as the substrate, the activity level of multi-copper oxidase was measured to be 4.63U / L. Add 2U / L crude enzyme solution to biogenic am...
Embodiment 2
[0049] Example 2: Cloning, expression and purification of multi-copper oxidase gene
[0050] (1) Cloning and expression of multi-copper oxidase gene
[0051] Using the genomic DNA of Lactobacillus sakei preserved in our laboratory as a template, the target gene was amplified, and the amplified product fragments were amplified, as shown in the electrophoresis figure 1 As shown, the band size is about 1500bp, and the nucleotide sequence is shown in SEQ ID NO.2.
[0052] The amplified fragment was connected between the Sac I enzyme and the Xho I enzyme cutting site of the plasmid pET-28a to construct a recombinant vector. The recombinant vector was transformed into E.coli DH5α, cultivated, and the plasmid was extracted for screening and sequencing, and it was confirmed that the recombinant vector with the correct coding frame was obtained.
[0053] Extract the recombinant plasmid with correct sequencing and transform it into Escherichia coli BL21(DE3) competent cells. The trans...
Embodiment 3
[0057] Example 3: Degradation effect of recombinant multi-copper oxidase on histamine under different conditions
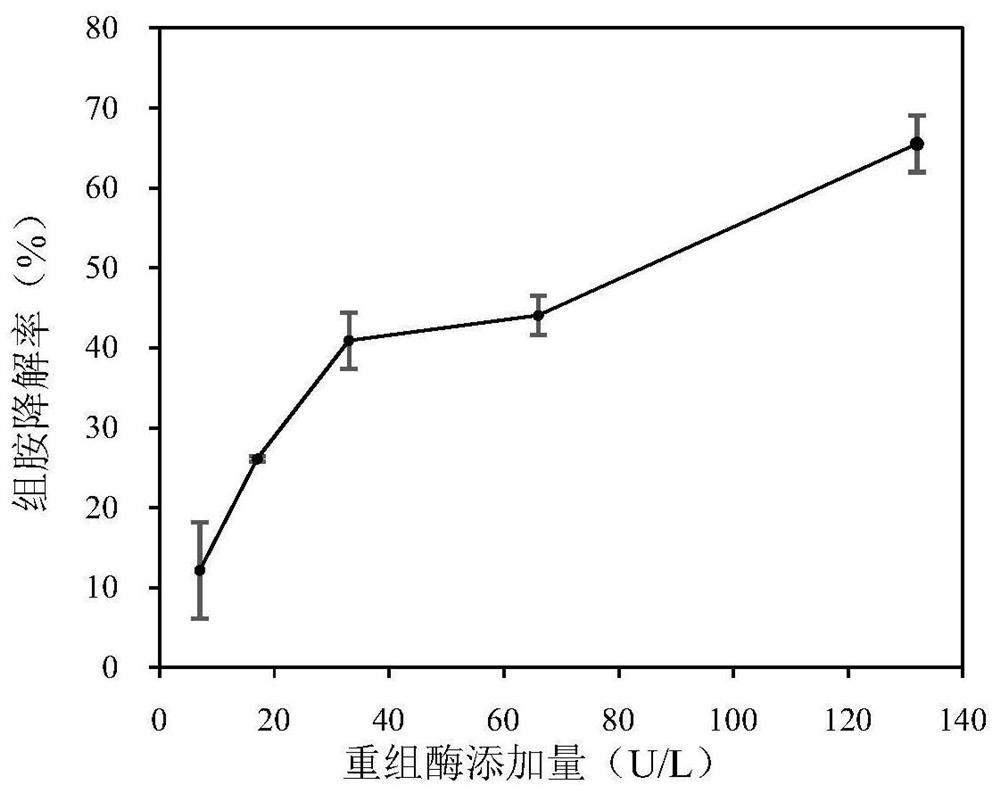
[0058] (1) Effect of the amount of recombinant multi-copper oxidase added on the degradation rate of histamine
[0059] The reaction system is a phosphate buffer solution with pH 7.0, and the final content of histamine is 100 mg / L; the purified recombinant enzyme Ls1b is added in different amounts (7U / L, 17U / L, 33U / L, 66U / L, 132U / L, L) adding to the reaction system; reacting at 25° C. for 24 hours; measuring the content of biogenic amine by ultra-high performance liquid chromatography-mass spectrometry. Histamine degradation results as figure 2 As shown, with the increase of the recombinant enzyme Ls1b content, the degradation rate of histamine increased from 12.16% to 65.5% within 24 hours.
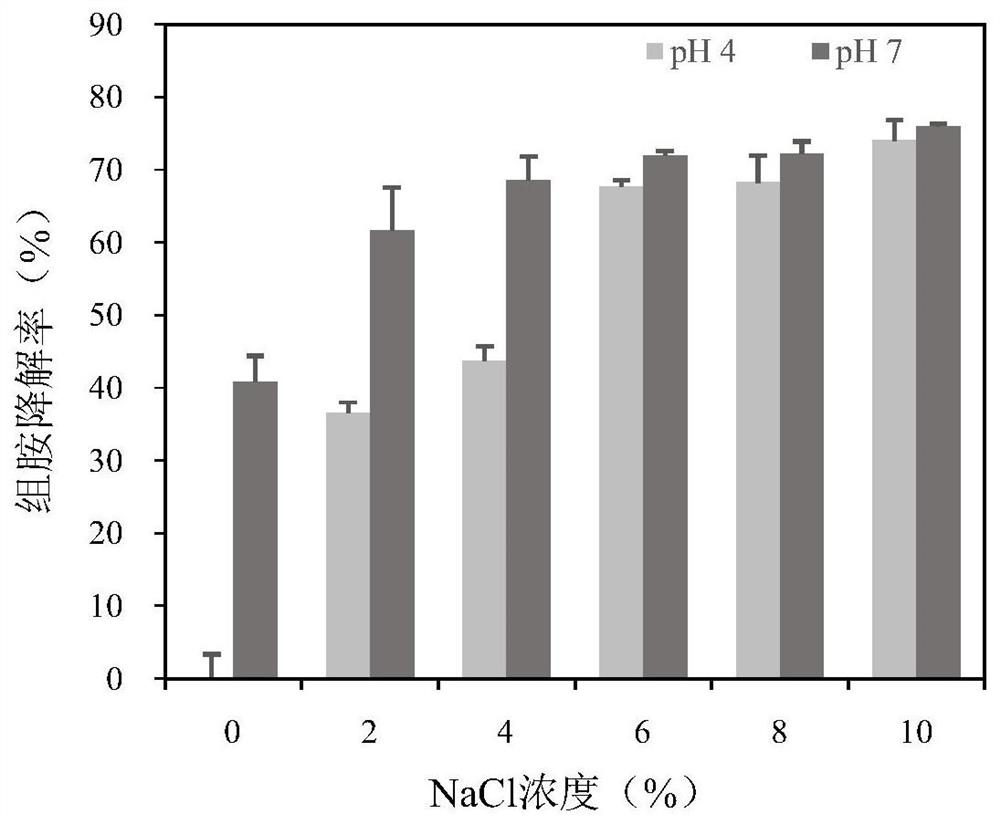
[0060] (2) Effect of salinity and pH on the degradation rate of recombinant multi-copper oxidase on histamine
[0061] The reaction system is a phosphate buffer (pH 4.0 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com