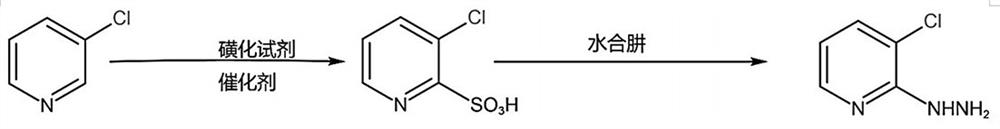
Synthesis method of 3-chloro-2-hydrazinopyridine
A hydrazinopyridine and synthetic method technology, which is applied in the field of preparation of pharmaceutical and pesticide intermediates, can solve the problems of low product yield, high equipment requirements, and many steps, and achieve high product purity and yield, low equipment requirements, etc. High and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In specific implementation, such as figure 1 Shown, the synthetic method of 3-chloro-2-hydrazinopyridine of the present invention comprises the following steps:
[0033] The first step, in the reactor, add 3-chloropyridine, the first solvent, the catalyst and stir evenly, add the sulfonation reagent, heat up the reaction; cool down after the reaction, add the second solvent, continue to cool down and then filter, the filter cake Purify by vacuum distillation to obtain 3-chloro-2-pyridinesulfonic acid.
[0034] Wherein, the first solvent is a dialkyl sulfate; the second solvent is one of ether, trichloroethylene, tetrachloropropene or any mixture thereof; the catalyst is a heavy metal sulfate; the 3-chloropyridine, The equivalent ratio of the sulfonating reagent and the catalyst is 1: (1.1-5): (0.005-0.2); the weight ratio of the 3-chloropyridine, the first solvent, and the second solvent is 1: (2-10): ( 2~10).
[0035] Specifically, first raise the temperature to 60°...
Embodiment 1
[0042] Embodiment 1, the synthesis of intermediate 3-chloro-2-pyridinesulfonic acid
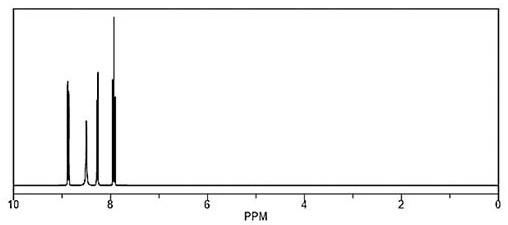
[0043] Add 56.5g of 3-chloropyridine, 150g of diethyl sulfate, HgSO 4 Start stirring at 1.5g, raise the temperature to 60°C, slowly add 100g of 65% oleum dropwise, after the drop, raise the temperature to 140°C-150°C, keep the temperature for 12 hours, take samples for detection, 3-chloropyridine HPLC is 1.28%, cool down To 20°C-25°C, add 200g of diethyl ether, continue to cool down to 0°C-5°C, stir for 2 hours, filter, and filter the cake through high vacuum distillation to obtain 82.5g of the intermediate compound, with a yield of 85.67%. The intermediate compound 3-chloro-2-pyridinesulfonic acid 1 H NMR 300MHz DMSO results as shown in figure 2 shown.
Embodiment 2
[0044] The synthesis of embodiment 2,3-chloro-2-hydrazinopyridine
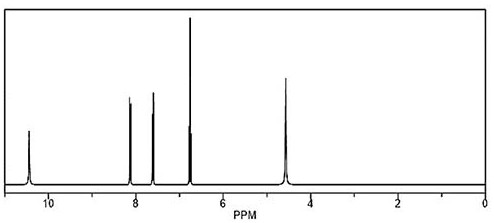
[0045] Add 96.7g of the intermediate 3-chloro-2-pyridinesulfonic acid, 300g of xylene, 10g of ethanolamine, 125g of 80% hydrazine hydrate into a 1000ml four-necked reaction flask, start stirring, raise the temperature to 140°C-150°C, keep it warm for 20 hours, and take a sample Detection, 3-chloro-2-hydrazinopyridine HPLC is 97.30%, then cooled to 0 ° C -10 ° C, stirred for 1 hour to start suction filtration, the filter cake was washed with 200g of water to obtain 3-chloro-2-hydrazinopyridine wet The finished product was dried with infrared light, weighed and tested to obtain 68.9g of finished product with a yield of 96.09%. The finished product 3-chloro-2-hydrazinopyridine 1 H NMR 300MHz DMSO results as shown in image 3 Shown; HPLC detection purity is 99.68%, and liquid phase chromatogram is as follows Figure 4 And shown in table 1, two-step reaction total yield is: 82.32%.
[0046] Table 1
[0047] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com