Method for synthesizing tetrahydronaphthalene-2-alcohol derivative compound
A technology of alcohol derivatives and compounds, which is applied in the field of compound preparation, can solve the problems of difficult acquisition of reaction raw materials, low yield of key intermediates, and harsh reaction conditions, and achieve the effects of high selectivity, simple route, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] A method for synthesizing tetralin-2-alcohol derivatives, comprising the steps of:
[0067] (1) Add compound I (133.8mg, 0.3mmol), palladium acetate (3.4mg, 0.015mmol), N-fluorobisbenzenesulfonamide (0.142g, 0.45mmol), 1,2- Dichloroethane (3mL), closed stirring reaction at 90°C for 24h, R in compound I 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 Both are hydrogen;
[0068] (2) Dilute the mixed solution obtained in step (1) with ethyl acetate (10mL), filter and remove the solvent under reduced pressure, and the residue is subjected to column chromatography [GF254 silica gel; 100-200 mesh; developer is V (petroleum ether) / V (ethyl acetate)=50 / 1] separation and purification, collecting the eluent containing the product, and distilling off the solvent from the eluent to obtain 115.9 mg of intermediate product II (yield 87%);
[0069] The obtained intermediate product II is carried out structural analysis, and the results are as follows:
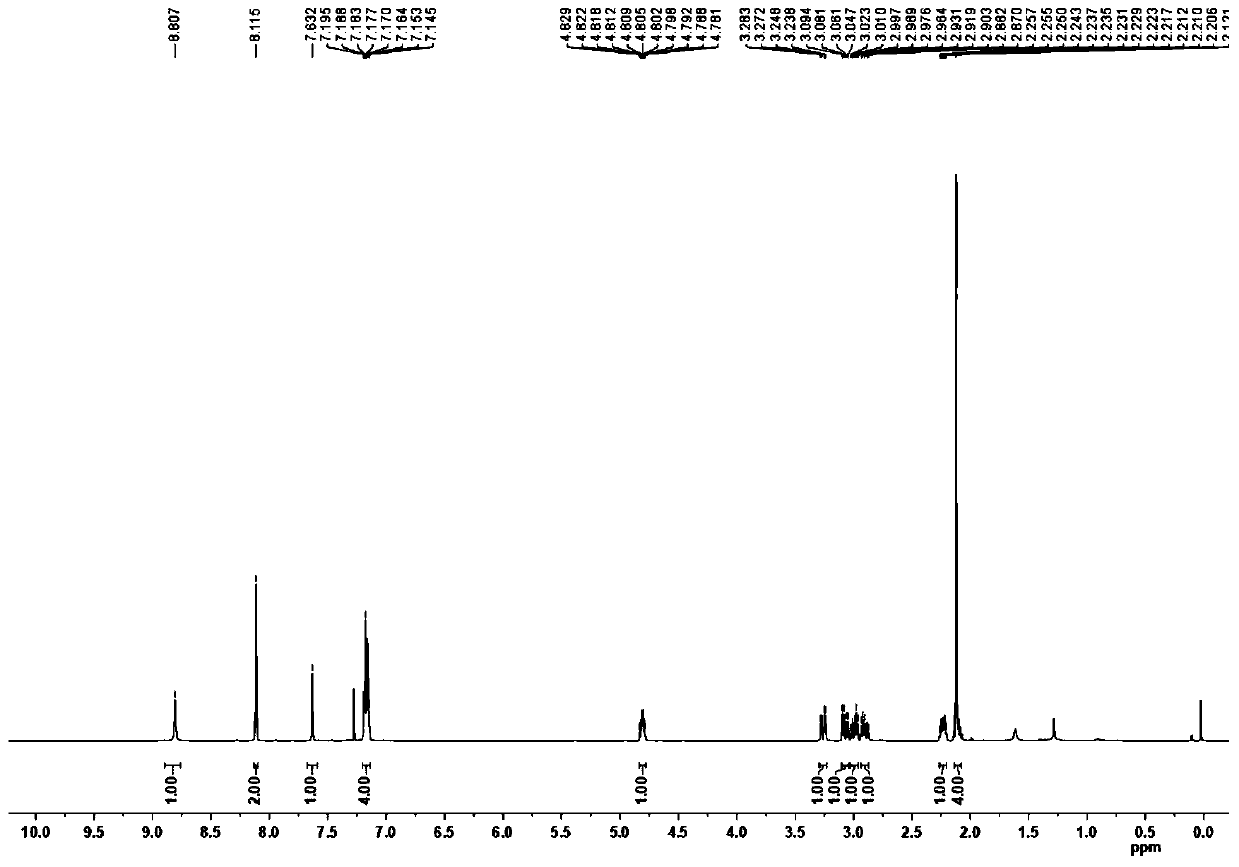
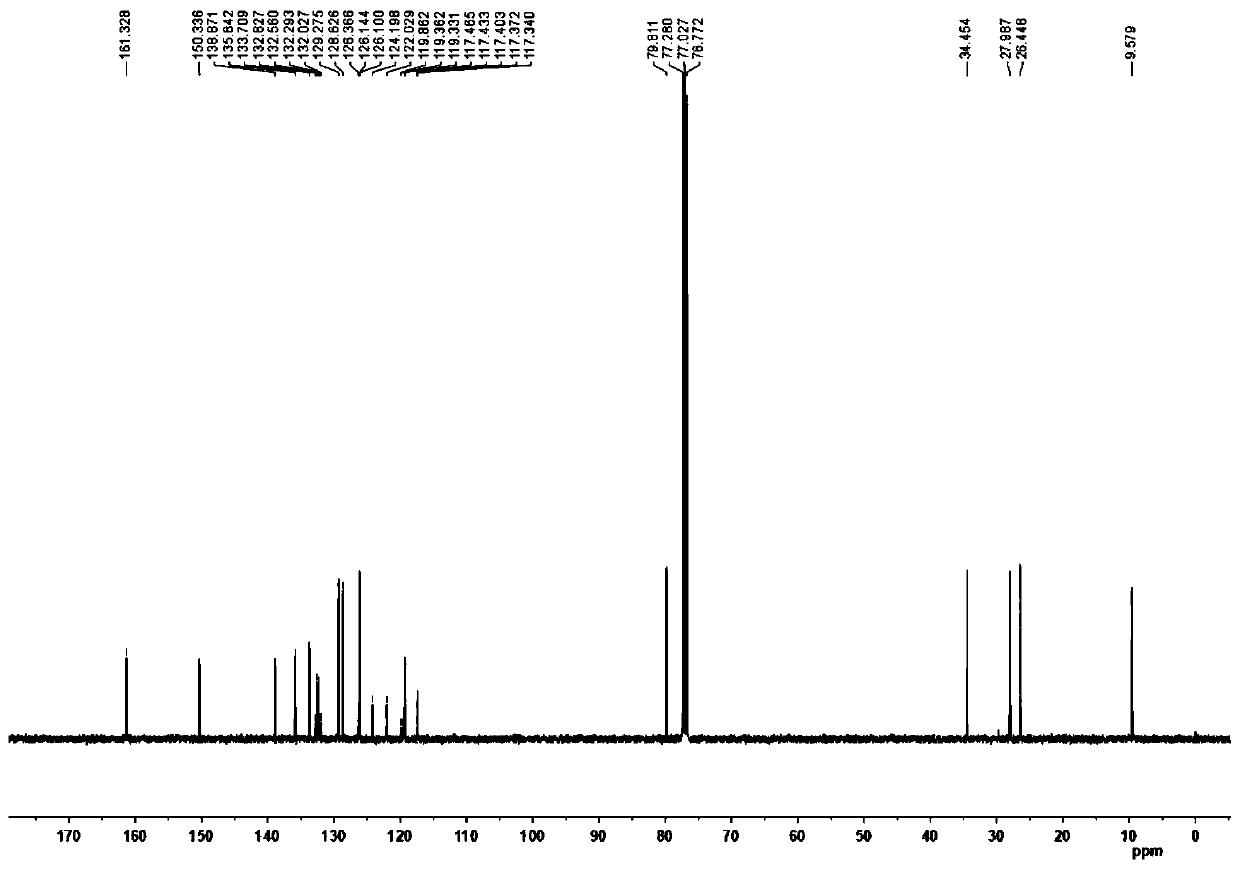
[0070] White solid; 1 H...
Embodiment 2
[0076] A method for synthesizing tetralin-2-alcohol derivatives, comprising the steps of:
[0077] (1) Add compound I (144.6mg, 0.3mmol), palladium chloride (5.4mg, 0.03mmol), N-fluorobisbenzenesulfonamide (0.142g, 0.45mmol), 1,2 -dichloroethane (3mL), closed stirring reaction at 90°C for 24h, R in compound I 1 , R 2 , R 5 , R 6 are hydrogen, R 3 , R 4 Connect to form a benzene ring, and form a naphthyl group together with the original benzene ring;
[0078] (2) Dilute the mixed solution obtained in step (1) with ethyl acetate (10mL), filter and remove the solvent under reduced pressure, and the residue is subjected to column chromatography [GF254 silica gel; 100-200 mesh; developer is V (petroleum ether) / V (ethyl acetate)=50 / 1] separation and purification, collecting the eluent containing the product, and distilling off the solvent from the eluent to obtain 112.3 mg of intermediate product II (yield 78%);
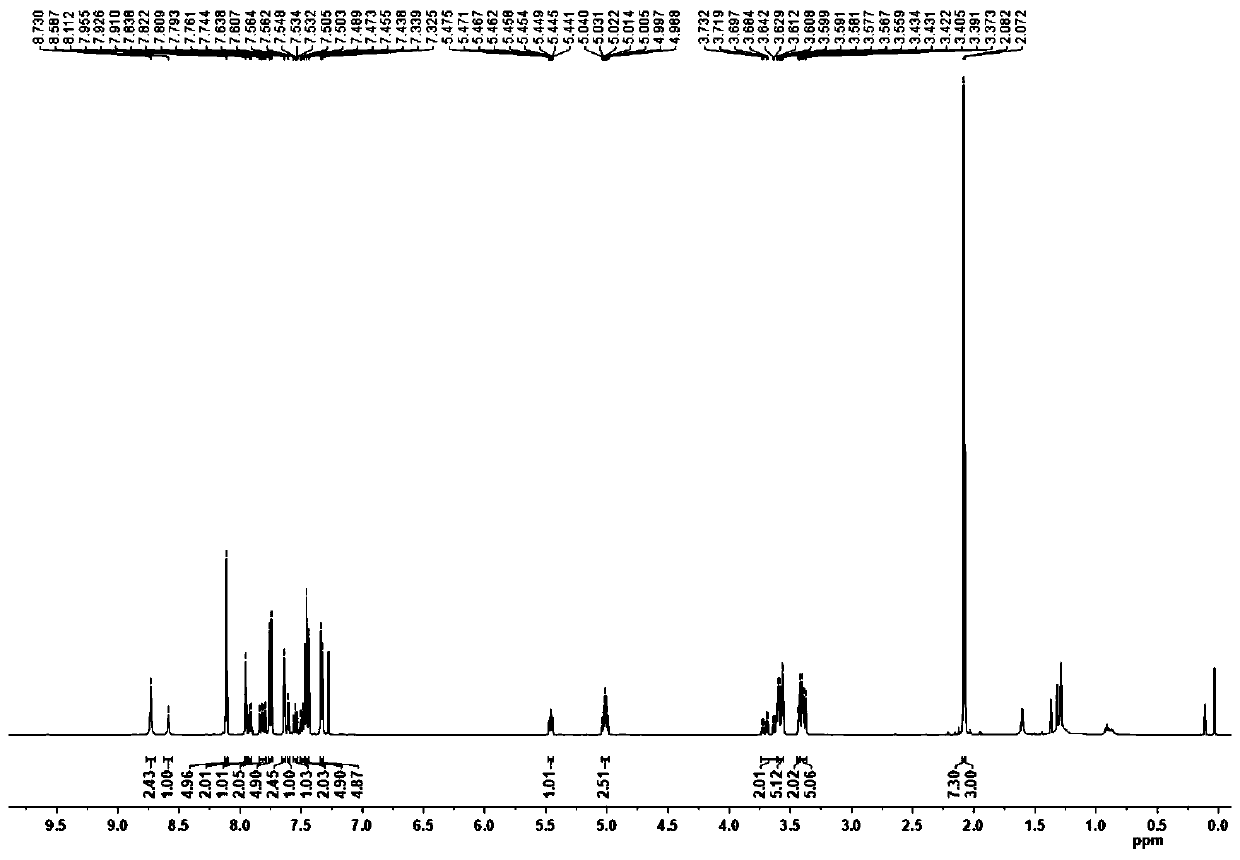
[0079] The obtained intermediate product II is carried out st...
Embodiment 3
[0087] A method for synthesizing tetralin-2-alcohol derivatives, comprising the steps of:
[0088] (1) Add compound I (156.6mg, 0.3mmol), palladium acetate (6.8mg, 0.03mmol), selectfluor (0.159g, 0.45mmol), 1,2-dichloroethane (3mL) in a closed reaction vessel , Stir the reaction at 90°C for 24h, the R in compound I 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 are hydrogen, R 8 is phenyl;
[0089] (2) Dilute the mixed solution obtained in step (1) with ethyl acetate (10mL), filter and remove the solvent under reduced pressure, and the residue is subjected to column chromatography [GF254 silica gel; 100-200 mesh; developer is V (petroleum ether) / V (ethyl acetate)=50 / 1] separation and purification, collecting the eluate containing the product, and distilling off the solvent from the eluent to obtain 120.1 mg of intermediate product II (yield 77%);
[0090] The obtained intermediate product II is carried out structural analysis, and the results are as follows:
[0091] White s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com