Culture medium for helicobacter pylori as well as preparation method and application of culture medium
A Helicobacter pylori, Helicobacter pylori technology, applied in the field of microbiology, can solve the problems of no advantages, inability to adapt to the requirements of Helicobacter pylori isolation and culture, and low positive detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 This embodiment provides a medium for pylorus spiral bacilli and a preparation method thereof
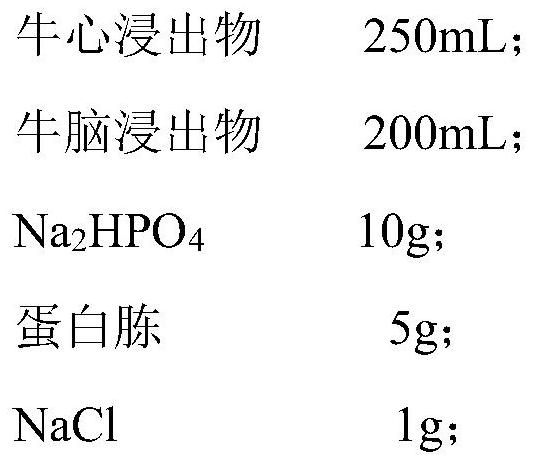
[0041] 1.1 Medium Formulation:
[0042] Agar 5g, cysteine 8g, antibiotic 0.5 mg, soluble starch 6g, 0.1 ml of lactic acid and horses 8g;
[0043] In the antibiotics, the mass fraction of vancomycin, amphotericin B and polymyxin is: vancomycin: 49%; amphotericin B: 49%; polymyxin: 2%.
[0044] 1.2 Reagent material source:
[0045] Joan: Beijing Luqiao Technology Co., Ltd.; cysteine, soluble starch: Annegi Chemical Co., Ltd .; 万, polyphenycin, amphotericin B: BIOSHARP; lactic acid: National Pharmaceutical Group; Ma Quan Blood: Jilin University Medical College Animal Center.
[0046] 1.3 Preparation method:
[0047] S1: Take 0.245 mg according to the formulation, amphotericin B 0.245 mg and polymyxin 0.01 mg dissolved in an appropriate amount of sterile water, mix well, irradiated under ultraviolet light, spare;
[0048] S2: According to the formulation quantity, solu...
Embodiment 2
[0050] Example 2 This example provides a medium of Helicobacterius and a preparation method thereof
[0051] 1.1 Medium Formulation:
[0052] Agar 5g, cysteine 8g, antibiotic 0.5 mg, soluble starch 6g, 0.1 ml of lactic acid, 3 mg of pylori rod bacteria lipopolysaccharide extract 3mg and horses 8g;
[0053]In the antibiotics, the mass fraction of vancomycin, amphotericin B and polymyxin is: vancomycin: 49%; amphotericin B: 49%; polymyxin: 2%.
[0054] 1.2 Preparation of Helicobacter bacteria lipopolysaccharide extract
[0055] First activation, screening, expanding cultivating, screening, and expanding cultivating, and collecting a bacterial suspension is collected in a bacterial suspension. The bacterial suspension was centrifuged by 5000 rpm for 10 min to precipitate. After washing with PBS buffer, 5000 rpm was centrifuged for 10 min, repeated 2 times. The precipitate (bacteria) was resuspended with PBS buffer, and the concentration of the bacteria was 2.0 × 10 10 A / ml, made ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com