Drug-loaded ceramic artificial bone with extremely small curved surface structure and preparation method of drug-loaded ceramic artificial bone
A very small curved surface, artificial bone technology, applied in the medical field, can solve the problems of poor compatibility of artificial bone, side effects of anti-inflammatory drugs, etc., and achieve the effect of improving the success rate of surgery, facilitating proliferation, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
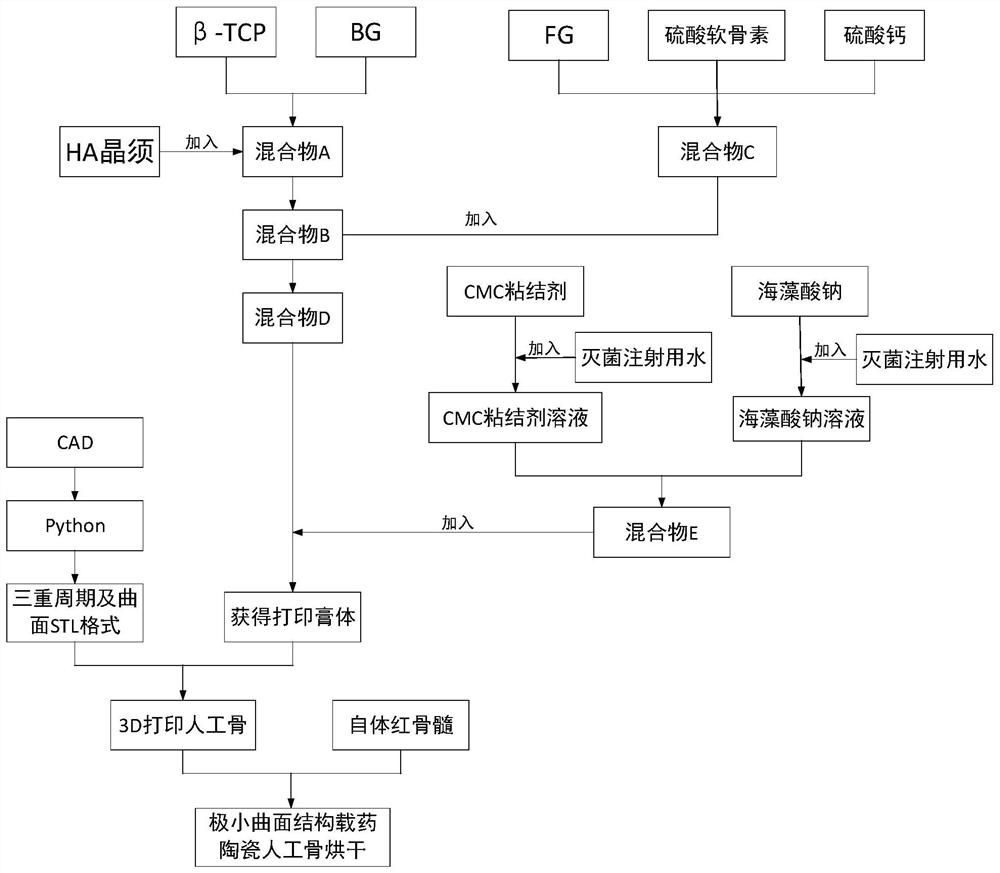
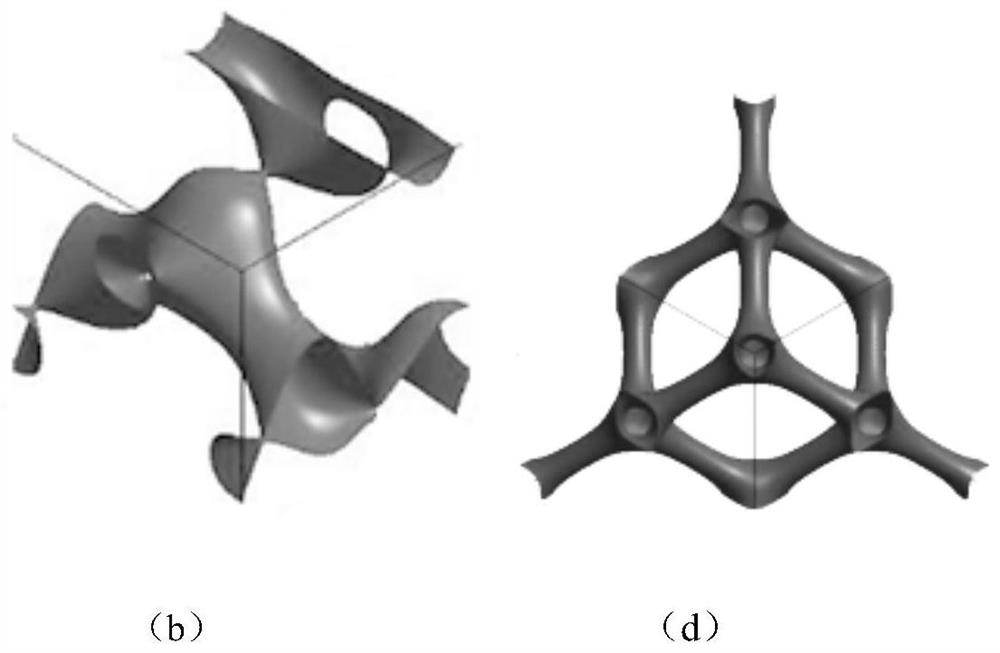
[0032] The invention provides a formula and a preparation method of a drug-loaded ceramic artificial bone with an extremely small curved surface structure. The preparation method of the artificial bone includes: providing raw materials, the raw materials including bioactive glass (BG), β-tricalcium phosphate, HA Whiskers, calcium sulfate, fibrinogen (FG), carboxymethylcellulose (CMC), sodium alginate, chondroitin sulfate, autologous red bone marrow. The method of preparation is to combine composite materials with triple periodic minimal surfaces.
[0033] see figure 1 , the preparation method of artificial bone is as follows:
[0034] (1) Preparation of binder: Weigh carboxymethylcellulose (CMC) and dissolve it in sterile water for injection, place it in a temperature-controlled magnetic stirrer at 85°C and stir for 3 hours, 5% to 6% of CMC will bind agent.
[0035] (2) Preparation of sodium alginate solution: Weigh sodium alginate and dissolve it in sterile water for injec...
Embodiment example 1
[0055] (1) Preparation of binder: Weigh 3g of CMC and 47g of sterilized water for injection and pour it into a blue cap bottle to prepare a 6% CMC solution, place it in a temperature-controlled magnetic stirrer at 85°C and stir for 3 hours to prepare into a 6% CMC solution.
[0056] (2) Weigh sodium alginate and dissolve it in sterile water for injection, place it in a water bath at 55°C and stir slowly for 2 hours, and completely dissolve into a 2% homogeneous solution.
[0057] (3) Preparation of printing paste: mix BG and bioceramic β-tricalcium phosphate in a mass ratio of 5:5 to obtain mixture A; then add HA whiskers to mixture A to obtain mixture B, and HA whiskers are added to mixture B The mass fraction in is 5%; then calcium sulfate, fibrin coagulant and chondroitin sulfate are mixed according to the mass fraction ratio of 55%, 5%, and 40% to obtain mixture C; then the above-mentioned obtained mixture C is added to mixture B, and mixed to obtain Mixture D, wherein th...
Embodiment example 2
[0064] (1) Preparation of binder: Weigh 3.5g of CMC and 46.5g of sterile water for injection and pour them into a blue-capped bottle to prepare a 7% CMC solution, and stir in a temperature-controlled magnetic stirrer at 85°C for 3 hours , to make a 6% CMC solution.
[0065] (2) Weigh sodium alginate and dissolve it in sterile water for injection, place it in a water bath at 55°C and stir slowly for 2 hours, and completely dissolve into a 3% homogeneous solution.
[0066] (3) Preparation of printing paste: mix BG and bioceramic β-tricalcium phosphate in a mass ratio of 7:5 to obtain mixture A; then add HA whiskers to mixture A to obtain mixture B, and HA whiskers are added to mixture B The mass fraction in is 4%; then calcium sulfate, fibrinogen and chondroitin sulfate are mixed according to the mass fraction ratio of 60%, 3%, and 37% to obtain mixture C; then the above obtained mixture C is added to mixture B, and mixed to obtain Mixture D, wherein the mass fraction of mixtur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com