Efficient synthesis method of histamine H3 receptor antagonist pitolisant
The technology of a receptor antagonist and a synthesis method, which is applied in the field of synthesis of pharmaceutical compounds, can solve problems such as unfavorable pharmaceutical production and safe use, and achieve the effects of overcoming bottleneck problems and short reaction routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
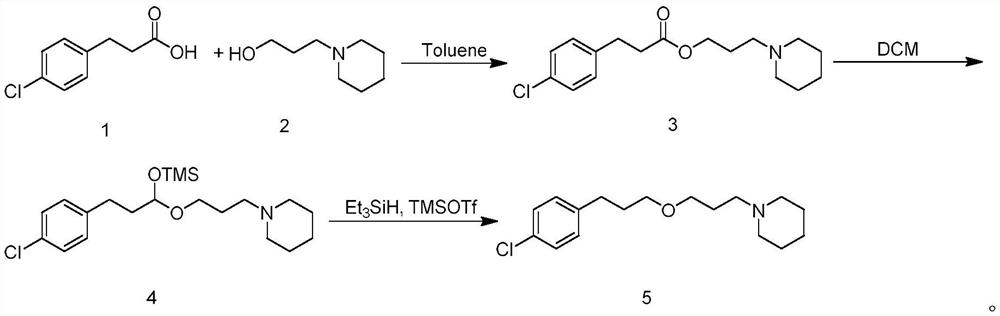
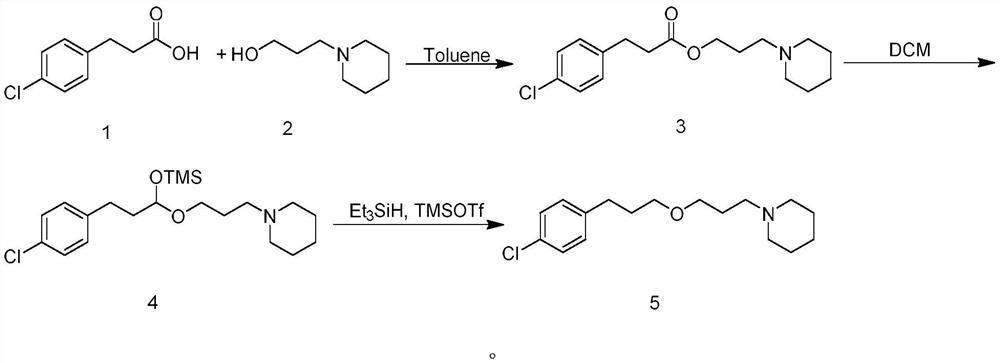
[0022] Preparation of 3'-(piperidine)-propyl-4-chlorophenylpropionate (compound 3)
[0023] Add toluene (150mL), 4-chlorophenylpropionic acid (20.2g, 0.11mol), 1-piperidinepropanol (14.3g, 0.1mol) and p-toluenesulfonic acid (2g) in a 250mL three-necked flask, heat to reflux water. After the reaction was complete, the temperature was lowered to room temperature, the reaction solution was washed twice with saturated sodium carbonate (50 mL), separated, the organic phase was dried over anhydrous sodium sulfate, the desiccant was filtered off, and the mother liquor was spin-dried to obtain 26 g of the target compound 3 with a yield of 84%. . 1 H-NMR: (400MHz, CDCl 3 )δ1.43(m,2H),1.58(m,4H),1.82(m,2H),2.36(t,6H),2.61(t,2H),2.93(t,2H),4.09(t,2H) ,7.14-7.23(m,4H).
Embodiment 2
[0025] Preparation of 3'-(piperidine)-propyl-4-chlorophenylpropionate (compound 3)
[0026] Add thionyl chloride (100 mL), 4-chlorophenylpropionic acid (20.2 g, 0.11 mol) and DMF (1 mL) into a 250 mL three-necked flask, heat to reflux for 2 hours, and distill under reduced pressure to remove the remaining thionyl chloride. Anhydrous tetrahydrofuran (100 mL) and 1-piperidine propanol (14.3 g, 0.1 mol) were added to the residue, and heated to reflux until the reaction was complete. The solvent tetrahydrofuran was distilled off under reduced pressure, ethyl acetate (100 mL) was added to the residue, saturated sodium carbonate (100 mL) was added after dissolving, stirred for 10 minutes, separated, the organic phase was dried over anhydrous sodium sulfate, the desiccant was filtered off, and the mother liquor Spin-dried to obtain 25 g of the target product with a yield of 81%. 1 H-NMR: (400MHz, CDCl 3 )δ1.43(m,2H),1.58(m,4H),1.82(m,2H),2.36(t,6H),2.61(t,2H),2.93(t,2H),4.09(t,2H) ...
Embodiment 3
[0028] Preparation of 1-(3-(3-(4-chlorophenyl)-1-((trimethylsilyl)oxy)propoxy)propyl)piperidine (Compound 4)
[0029] Put DCM (50 mL) and compound 3 (3.1 g, 0.01 mol) in a three-neck flask under nitrogen protection, and cool to -78°C. Add DIBAL-H (12 mL, 1.0 M hexane dispersion) dropwise, and control the rate of addition so that the temperature of the mixed system is not higher than -70°C. After completion of the dropwise addition, control the temperature at -70°C to -78°C and stir for 30 minutes. Trimethylsilimidazole (4.2 g, 0.03 mol) was added dropwise and stirred for 1 hour. Add saturated NH dropwise 4 Aqueous Cl (30 mL) quenched the reaction. The quenched solution was naturally warmed to room temperature, filtered, and the mother liquor was separated. The aqueous phase was extracted three times with DCM (15 mL), and the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to give 3.6 g of crude compound 4. The compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com