Camelinine A derivative as well as preparation and application thereof in prevention and treatment of plant virus and pathogenic bacterial diseases
A technology of camelamine and plant pathogens, applied in the field of agricultural protection, can solve the problems that cytotoxicity is not directly related
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
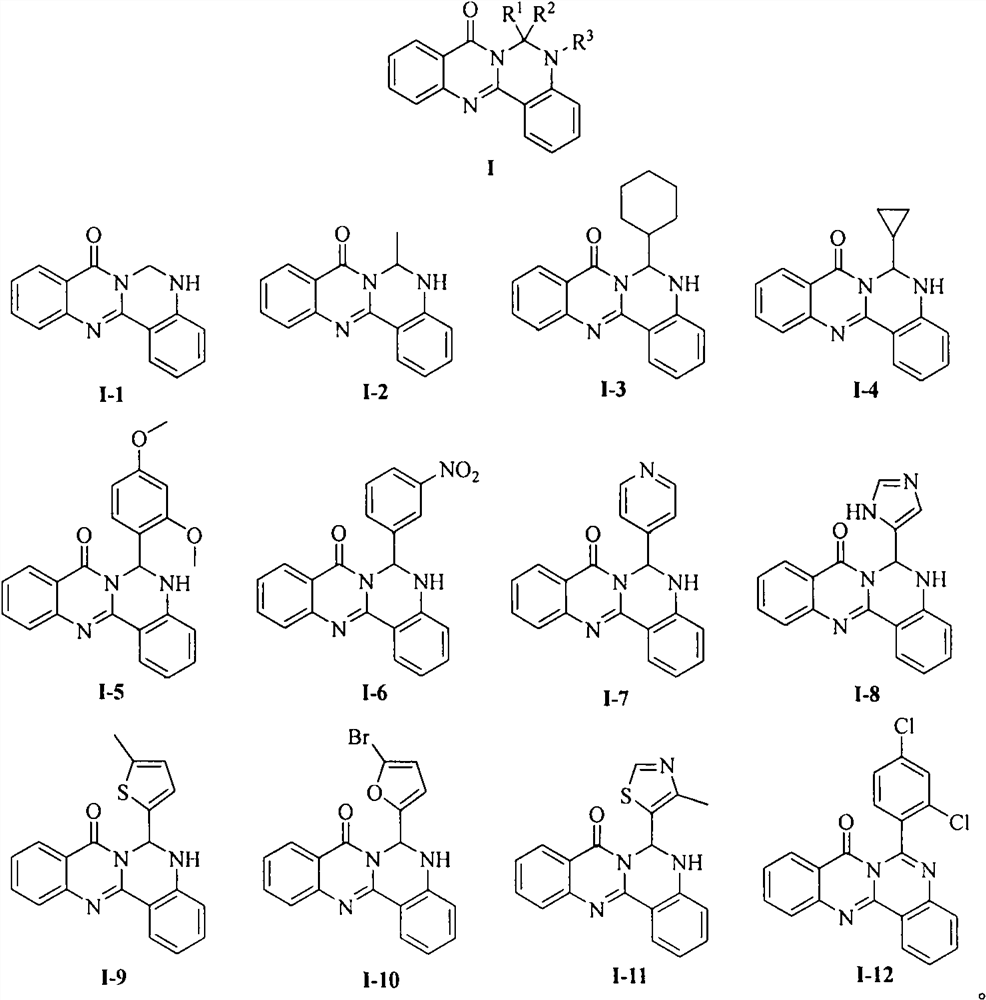
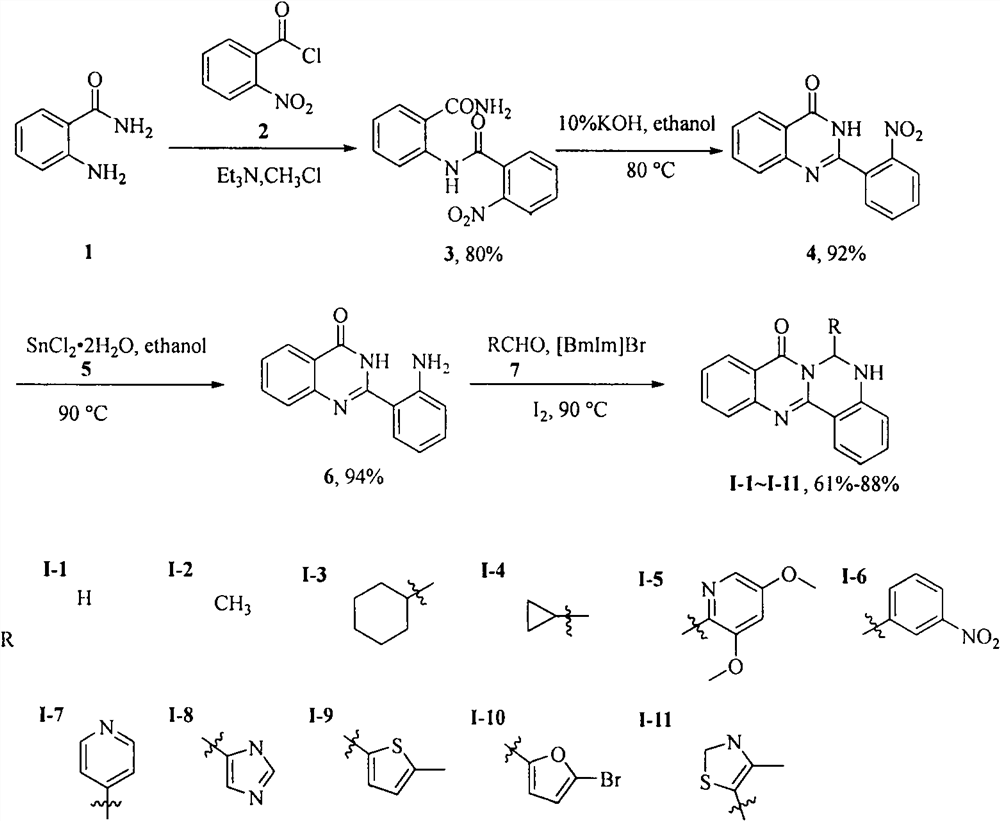
[0014] Embodiment 1: the synthesis of cameline A derivative I-1~I-12
[0015] The first step, the synthesis of intermediate 3. Dissolve anthranilamide (1) (5g, 37mmol) in chloroform, add triethylamine (14mL), slowly add o-nitrobenzoyl chloride (2) (7mL, 52mmol) under stirring, and react at room temperature for four hours , TLC detects that the reaction is complete, and suction filtration under reduced pressure gives 4.2 g of a white solid, with a yield of 80%, and a melting point of 195-197 ° C. 1 H NMR (400MHz, DMSO-d 6 )δ12.52(s, 1H), 8.48(d, J=8.2Hz, 1H), 8.38(brs, 1H), 8.11(d, J=8.1Hz, 1H), 7.92-7.75(m, 5H), 7.59(t, J=7.7Hz, 1H), 7.23(t, J=7.6Hz, 1H).
[0016] The second step is the synthesis of intermediate 4. Intermediate 3 (4g, 14mmol) was dissolved in 10% KOH aqueous solution (40mL) and absolute ethanol (20mL), heated to reflux at 90°C, TLC detected that the reaction was complete, most of the solvent was spin-dried, and extracted with ethyl acetate , dried over an...
Embodiment 2
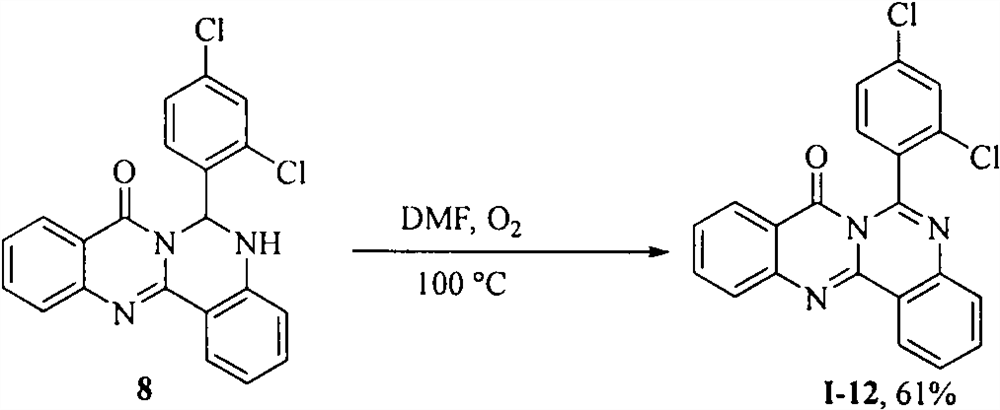
[0030] Embodiment 2: the synthesis of cameline A derivative I-12
[0031] Compound 8 (600mg, 2mmol) was dissolved in THF, protected by oxygen, heated at 100°C by TLC to detect the completion of the reaction, diluted with water, extracted with methyl tert-butyl ether, dried with anhydrous sodium sulfate, filtered with suction, precipitated under reduced pressure, column Chromatography (V (petroleum ether): V (ethyl acetate) = 10: 1) gave 480 mg of a light yellow solid with a yield of 61% and a melting point of 131-133°C. 1 H NMR (400MHz, CDCl 3 )δ8.82(d, J=7.9Hz, 1H), 8.23(d, J=8.0Hz, 1H), 7.87-7.76(m, 4H), 7.65(t, J=6.8Hz, 1H), 7.59( d, J=7.9Hz, 1H), 7.50-7.38(m, 3H). 13 C NMR (100MHz, CDCl 3 )δ159.8, 146.9, 146.6, 145.3, 142.0, 136.1, 135.7, 135.4, 133.7, 131.9, 129.8, 129.3, 129.0, 128.1, 127.6, 127.5, 127.3, 126.7, 126.2, 121.7, 119 for C 21 h 12 Cl 2 N 3 O[M+H] + 392.0352, found 392.0353.
Embodiment 3
[0032] Embodiment 3: the assay of anti-tobacco mosaic virus activity, assay procedure is as follows:
[0033] 1. Virus purification and concentration determination:
[0034] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0035] 2. Compound solution preparation:
[0036] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0037] 3. In vivo protection:
[0038] Select Shanxi tobacco with uniform growth at the 3-5 leaf stage, spray the whole plant, and repeat each treatment 3 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com