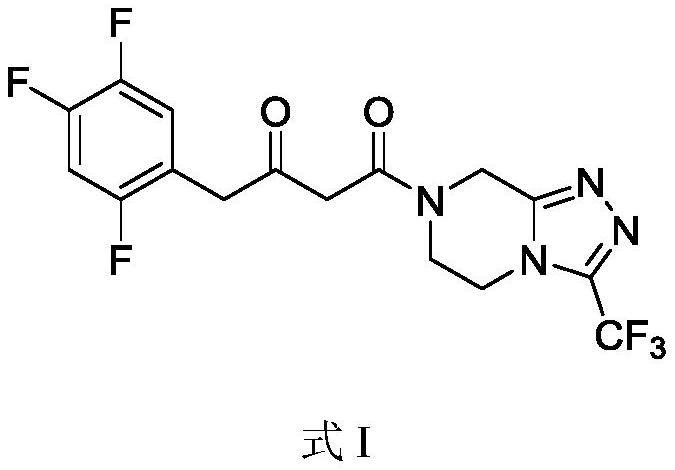
Recovery method of sitagliptin phosphate key intermediate
A technology of sitagliptin phosphate and recovery method, which is applied in the field of preparation of sitagliptin phosphate, and can solve problems such as difficult effective separation and recovery of key intermediates of sitagliptin phosphate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The HPLC content of the key intermediate of sitagliptin phosphate in the mother liquor is 62% (calculated by the external standard method, the concentration of the key intermediate of sitagliptin phosphate is 77mg / L), and the HPLC content of sitagliptin phosphate is 15%.
[0039] Add 10L of mother liquor (about 9 kg) into the reaction kettle, evaporate the solvent under the conditions of 50°C and vacuum degree ≥ 0.08MPa, and obtain a sticky substance after the solvent is recovered under reduced pressure. Add 10L of water and 10L of ethyl acetate to the sticky substance, stir and separate the liquids to obtain an aqueous phase and an organic phase, and then extract the aqueous phase with 10L of ethyl acetate to obtain an organic phase, and combine the obtained organic phases to be the extract. The solvent in the extract was distilled off at 50°C and vacuum degree ≥0.08MPa to obtain 1.20 kg of solid residue. In the solid residue, the HPLC content of the key intermediate of...
Embodiment 2
[0041] The mother liquor of embodiment 2 is identical with the mother liquor of embodiment 1.
[0042] Add 10L of mother liquor into the reaction kettle, evaporate the solvent under the conditions of 50°C and vacuum degree ≥ 0.08MPa, and obtain a sticky substance after the solvent is recovered under reduced pressure. Add 10L of water and 10L of dichloromethane to the sticky substance, stir and separate the liquids to obtain an aqueous phase and an organic phase, then extract the aqueous phase with 10L of dichloromethane to obtain an organic phase, and combine the obtained organic phases to be the extract. The solvent in the extract was distilled off at 50°C and vacuum ≥0.08MPa to obtain 1.34kg of solid residue. In the solid residue, the HPLC content of the key intermediate of sitagliptin phosphate was 80%. Phosphoric acid The HPLC content of sitagliptin was 2%. Add 8.00kg of 75% ethanol aqueous solution to the solid residue, and stir and crystallize at 10°C, and filter after ...
Embodiment 3
[0044] The mother liquor of embodiment 3 is identical with the mother liquor of embodiment 1.
[0045] Add 10L of mother liquor into the reaction kettle, evaporate the solvent under the conditions of 50°C and vacuum degree ≥ 0.08MPa, and obtain a sticky substance after the solvent is recovered under reduced pressure. Add 10L of water and 10L of dichloromethane to the sticky substance, stir and separate the liquids to obtain an aqueous phase and an organic phase, then extract the aqueous phase with 10L of dichloromethane to obtain an organic phase, and combine the obtained organic phases to be the extract. The solvent in the extract was distilled off at 50°C and vacuum degree ≥0.08MPa to obtain 1.20 kg of solid residue. In the solid residue, the HPLC content of the key intermediate of sitagliptin phosphate was 83%. Phosphoric acid HPLC content of sitagliptin 3%. Add 20.00kg of 70% methanol aqueous solution to the solid residue, and stir and crystallize at 20°C. After stirring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com