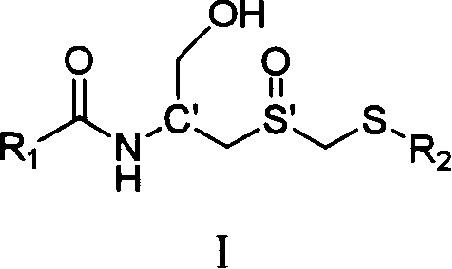
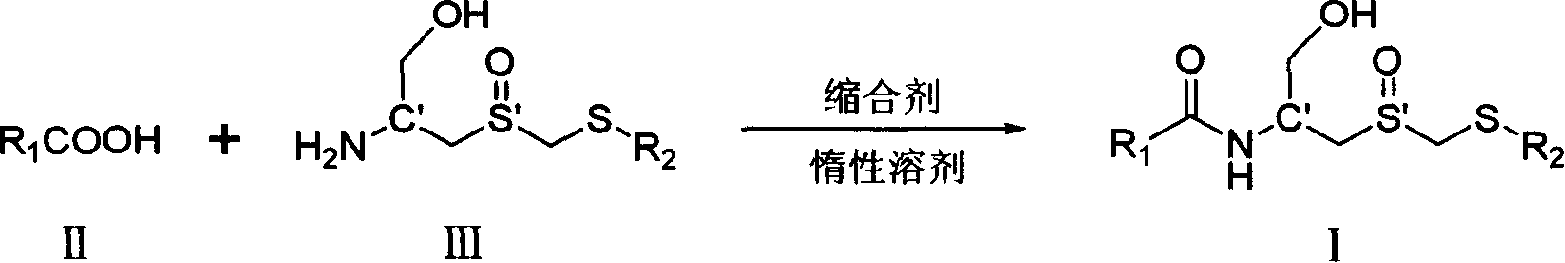
Carboxyl acylamide compound and its preparing method and use
A technology of carboxamides and compounds is applied in the field of carboxamide fungicides and their preparation, and can solve the problems of unfriendly environment of fungicides, toxic and side effects of humans and animals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Preparation of (Rc, Ss)-(1-hydroxymethyl-2-methylsulfoxide ethyl)-5-methylpyrazine-2-amide (I-1)
[0098] In a 50 mL round-bottomed flask, add (Rc, Ss)-2-amino-3- Methylsulfoxide propanol (0.5mmol, 92mg, the compound was prepared according to the literature J.Org.Chem.1981,46,5408-5413), dicyclohexylcarbodiimide (114mg, 0.55mmol), 1- Hydroxybenzotriazole (5 mg), stirred at room temperature for 24 hours. After the solvent was removed under reduced pressure, 123 mg of white solid was obtained by column chromatography, with a yield of 80.9%; 1 H NMR (400MHz, D 2 O) δ8.97(s, 1H, Py), 8.56(s, 1H, Py), 4.64(m, 1H, NHCH), 4.08 and 3.91(AB spectrum, d, J=14.0Hz, 2H, SOCH 2 S), 3.80 (m, 2H, CH 2 OH), 3.31-3.18 (m, 2H, SOCH 2 CH), 2.60(s, 3H, PyCH 3 ), 2.21(s, 3H, SCH 3 ); 13 C NMR (100MHz, D 2 O+CD 3 OD-d 4 )δ 165.84, 158.08, 144.34, 142.12, 141.60, 63.03, 54.77, 52.52, 46.95, 20.74, 16.19.
Embodiment 2
[0099] Example 2: Preparation of (Rc, Rs)-(1-hydroxymethyl-2-methylsulfoxide ethyl)-5-methylpyrazine-2-amide (I-2)
[0100] In a 50 mL round-bottomed flask, add (Rc, Rs)-2-amino-3- Methylsulfoxide propanol (0.5mmol, 92mg, the compound was prepared according to the literature J.Org.Chem.1981,46,5408-5413), dicyclohexylcarbodiimide (114mg, 0.55mmol), 1- Hydroxybenzotriazole (5 mg), stirred in an ice-water bath for 24 hours. After the solvent was removed under reduced pressure, 112 mg of a colorless oil was obtained by column chromatography, with a yield of 73.6%; 1 H NMR (300MHz, D 2 O) δ8.99 (s, 1H, Py), 8.60 (s, 1H, Py), 4.63 (m, 1H, NHCH), 4.16 and 3.96 (AB spectrum, d, J=13.9Hz, 2H, SOCH 2 S), 3.85 (m, 2H, CH 2 OH), 3.44 and 323 (ABpart of ABX spectrum, 8 lines, J AB =13.7Hz,J AX =8.7Hz,J BX =5.1Hz, 2H, SOCH 2 CH), 2.63(s, 3H, PyCH 3 ), 2.27(s, 3H, SCH 3 ); 13 C NMR (75.5MHz, D 2 O+CD 3 OD-d 4 )δ 166.47, 159.04, 145.30, 143.01, 142.58, 63.60, 55.80, 53.08, 48.62,...
Embodiment 3
[0101] Example 3: Preparation of (Sc, Ss)-(1-hydroxymethyl-2-methylsulfoxide ethyl)-5-methylpyrazine-2-amide (I-3)
[0102] In a 50 mL round-bottomed flask, (Sc, Ss)-2-amino-3- Methylsulfoxide propanol (0.5mmol, 92mg, the compound was prepared according to the literature J.Org.Chem.1981,46,3273-3283), dicyclohexylcarbodiimide (114mg, 0.55mmol), 1- Hydroxybenzotriazole (5 mg), stirred at room temperature for 24 hours. After the solvent was removed under reduced pressure, 131 mg of a colorless oil was obtained by column chromatography, with a yield of 73.6%; 1 H NMR (300MHz, D 2 O) δ9.01 (s, 1H, Py), 8.61 (s, 1H, Py), 4.67 (m, 1H, NHCH), 4.18 and 3.98 (AB spectrum, d, J=13.9Hz, 2H, SOCH 2 S), 3.86(d, J=5.2Hz, 2H, CH 2 OH), 3.46 and 3.25 (AB part of ABX spectrum, 8 lines, J AB =13.7Hz,J AX =8.7Hz,J BX =5.1Hz, 2H, SOCH 2 CH), 2.65(s, 3H, PyCH 3 ), 2.29 (s, 3H, SCH 3 ); 13 C NMR (75.5MHz, D 2 O+CD 3 OD-d 4 )δ 165.53, 158.03, 144.26, 142.00, 141.52, 62.57, 54.75, 51.99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com