Imide analog compounds and its preparation method
A compound and imide technology, applied in the field of preparation of fungicides, can solve the problems of toxic and side effects, unfriendly environment of fungicides, etc., and achieve the effects of simple preparation method, good bactericidal spectrum, and low cost of synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
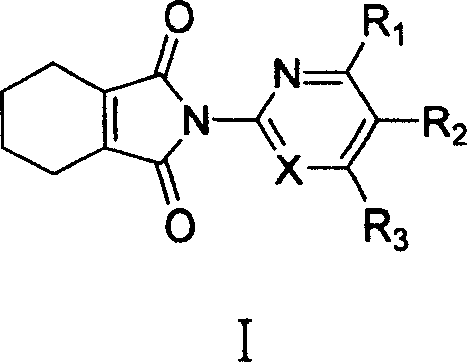
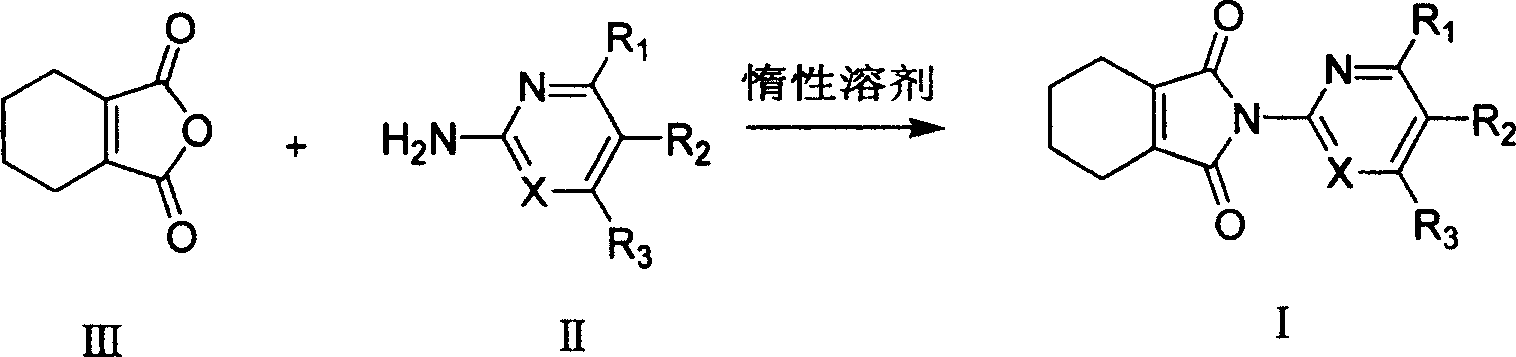
[0040] Example 1: Preparation of N-(4,6-bismethylpyrimidin-2-yl)-3,4,5,6-tetrahydrophthalimide (I-1)
[0041] Weigh 152 mg (about 1 mmol) of 3,4,5,6-tetrahydrophthalic anhydride and place it in a 25 mL round bottom flask, add 10 mL of chloroform, and then add 123 mg of 2-amino-4,6-bismethylpyrimidine (about 1 mmol ). The reaction temperature was maintained at 80°C. Stir for 12 hours, column chromatography (40g silica gel H, 2% CH 3 OH / CH 2 Cl 2 elution) to obtain 171mg of white solid. Yield 62%. Its physical and chemical data are as follows: [ 1 H] NMR (400MHz, CDCl 3 ): δ / ppm; 6.991(s, 1H, H-Pyrimidine), 2.470(s, 6H, 2CH 3 ), 2.335 (m, 4H, 2CH 2 ), 1.725 (m, 4H, 2CH 2 ).Mp.141-143℃.
Embodiment 2
[0042] Example 2: Preparation of N-(6-methylpyridin-2-yl)-3,4,5,6-tetrahydrophthalimide (I-5)
[0043] Weigh 152 mg (about 1 mmol) of 3,4,5,6-tetrahydrophthalic anhydride into a 25 mL round bottom flask, add 10 mL of trimethylbenzene, and then add 108 mg of 2-amino-6-methylpyridine (about 1 mmol). The reaction temperature was maintained at 150°C. Stir for 10 hours, column chromatography (40g silica gel H, 2% CH 3 OH / CH 2 Cl 2 Elution) to obtain 97mg of white solid. Yield 40%. Its physical and chemical data are as follows: [ 1 H] NMR (400MHz, CDCl 3 ): δ / ppm; 7.703-7.664 (t, J 1 =7.750Hz,J 2 =7.731Hz, 1H, Py), 7.172-7.153(d, J=7.667Hz, 1H, Py), 7.018-6.998(d, J=7.787Hz, 1H, Py), 3.033-3.011(m, 2H, 2CH ), 2.545 (s, 3H, CH 3 ), 1.885 (br.s, 4H, 2CH 2 ), 1.478-1.471 (m, 4H, 2CH 2 ).Mp.92-94°C.
Embodiment 3
[0044] Example 3: The reaction temperature was kept at 110° C., and 10 mL of chloroform was added instead of 10 mL of toluene. The other reaction conditions were the same as in Example 1, the yield was 63%, and the physical and chemical data were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com