Method for separating and detecting rifampicin and related impurities in rifampicin for injection
A technology for rifampicin and injection, which is applied in the field of analytical chemistry, can solve the problems that cannot be separated at the same time, and cannot be used for the separation and detection of 14 kinds of impurities and rifampicin at the same time, so as to achieve precise quality control, improve efficiency and accurate detection results. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Chromatographic conditions:
[0069] Use octylsilane bonded silica gel as filler (Agilent ZORBAX Eclipse XDB-C8 4.6mm×250mm, 5μm), and buffer saline solution (take 8.67g of potassium dihydrogen phosphate and 30g of citric acid monohydrate, add water to dissolve and dilute to 1000ml)-methanol-acetonitrile (46:29:25) as mobile phase A, using glacial acetic acid methanol solution (take 5ml of glacial acetic acid, add methanol 1000ml, shake well) as mobile phase B, and perform linear gradient elution in the following table. The detection wavelength is 254nm, the flow rate is 1.4ml per minute, and the column temperature is 30°C.
[0070] Gradient elution program:
[0071]
Embodiment 2
[0073] Reagent preparation:
[0074] Blank solution: acetonitrile-water (1:1)
[0075] System Suitability Solution 1: Take an appropriate amount of impurity D, impurity I and rifampin reference substance, add a small amount of acetonitrile to dissolve, then quantitatively dilute with acetonitrile-water (1:1) to make a mixed solution containing about 40 μg per 1ml , as System Suitability Solution 1.
[0076] System Suitability Solution 2: Take an appropriate amount of impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, impurity K, impurity L and rifampicin, add a small amount of acetonitrile to dissolve , and then quantitatively diluted with acetonitrile-water (1:1) to prepare a mixed solution containing rifampicin 1mg / ml, as system suitability solution 2.
[0077] Sensitivity solution: Accurately weigh an appropriate amount of rifampicin reference substance, add a small amount of acetonitrile (about 10mg plus 1ml aceton...
Embodiment 3
[0081] Detection:
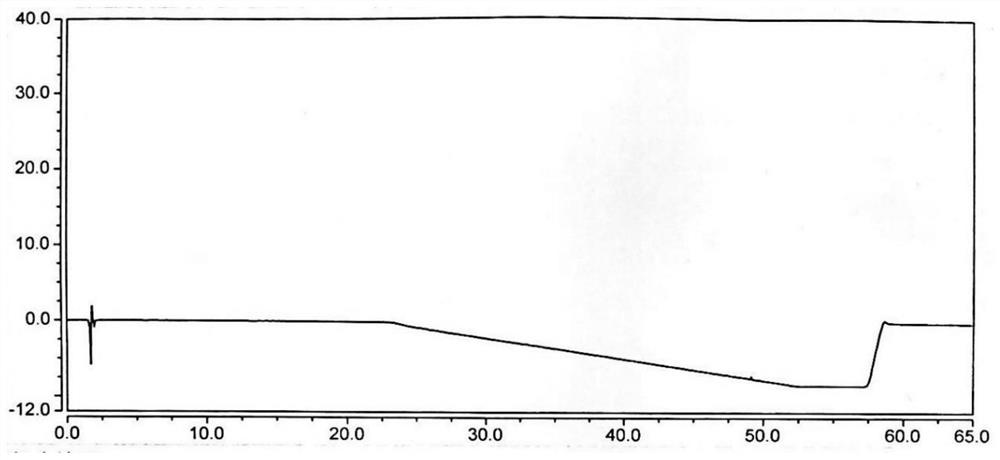
[0082] Take 10 μl of the blank solution and inject it into the liquid chromatograph, and record the chromatogram (see attached figure 1 ).
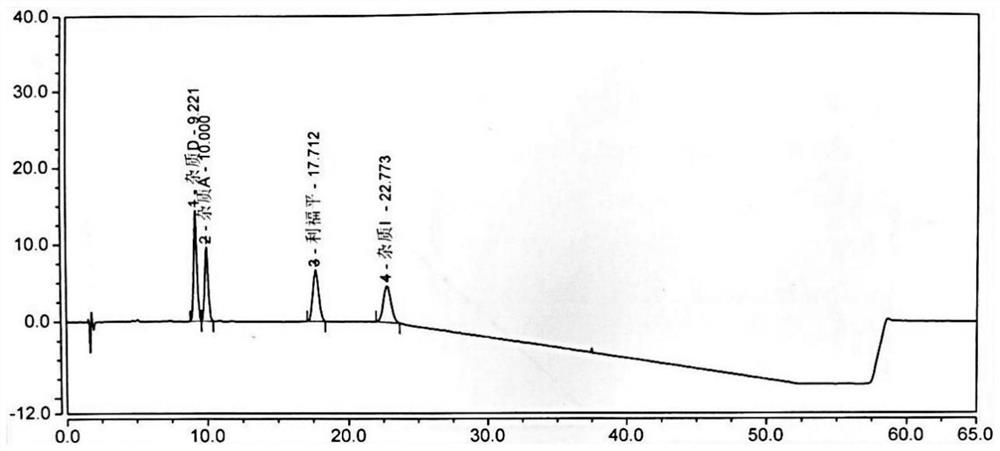
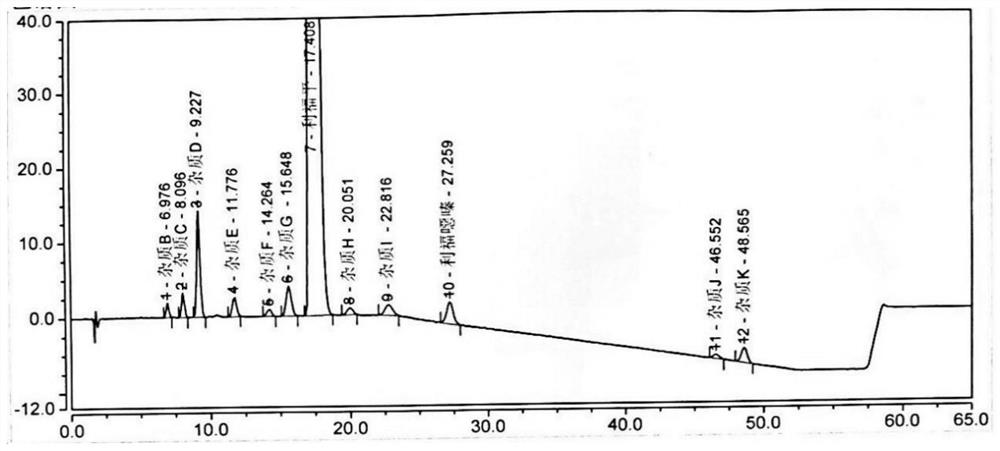
[0083] Take 10 μl each of system suitability solutions 1 and 2, inject them into the liquid chromatograph, and record the chromatograms. The separation degree of impurity D and impurity A (the impurity produced by impurity D and impurity I in this solution) meets the requirements (see attached figure 2 , 3 ).
[0084] Get 10 μ l of the sensitivity solution and inject it into the liquid chromatograph, record the chromatogram, the signal-to-noise ratio of the main component chromatographic peak height should be greater than 10 (see attached Figure 4 ).
[0085] Accurately measure 10 μl each of the test solution and the reference solution, inject them into the liquid chromatograph respectively, and record the chromatogram. If there are impurity peaks in the chromatogram of the test solution, except for the solvent p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com