A method for detecting related substances in ezetimibe rosuvastatin calcium tablets
A technology related to substances and detection methods, applied in the field of pharmaceutical analysis, to achieve the effects of high sensitivity, good accuracy, and simple mobile phase preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
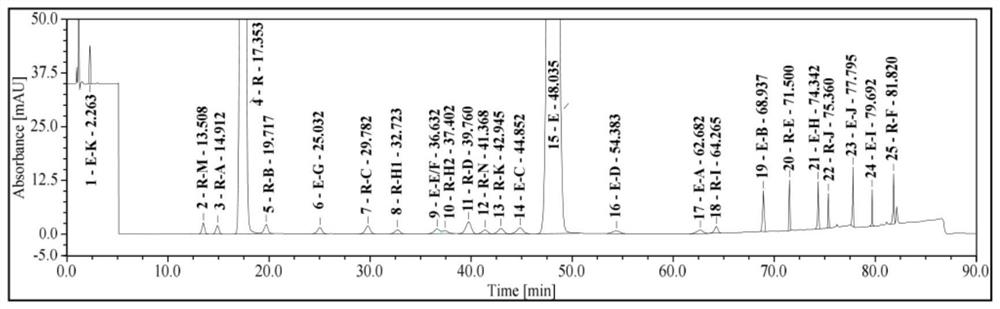
[0038](1) Sample preparation: take 5 tablets of ezetimibe rosuvastatin calcium tablets, put them in a 50ml measuring bottle, add 12ml of water-acetonitrile-acetic acid (40:60:0.1), ultrasonicate for 30min, cool to room temperature, add water - Dilute acetonitrile-acetic acid (40:60:0.1) to the mark, centrifuge for 5 minutes (10000 rpm), take the supernatant, filter it with a 0.45 μm PP filter head, discard 1 ml of the filtrate, and obtain.
[0039] (2) Reference substance solution: take about 10mg of ezetimibe reference substance and about 10mg (specification: 10mg / 10mg) or 5mg (specification: 10mg / 5mg) of rosuvastatin calcium reference substance, weigh them accurately, and place in 100ml volume In the bottle, add water-acetonitrile-acetic acid (40:60:0.1) to dilute to the mark, and shake well. Precisely measure 1ml, put it in a 100ml measuring bottle, add water-acetonitrile-acetic acid (40:60:0.1) to dilute to the mark, and shake well.
[0040] (3) Brand model of high perfor...
Embodiment 2
[0051] (1) Sample preparation: take 5 tablets of ezetimibe rosuvastatin calcium tablets, put them in a 50ml measuring bottle, add 12ml of water-acetonitrile-acetic acid (40:60:0.1), ultrasonicate for 30min, cool to room temperature, add water - Dilute acetonitrile-acetic acid (40:60:0.1) to the mark, centrifuge for 5 minutes (10000 rpm), take the supernatant, filter it with a 0.45 μm PP filter head, discard 1 ml of the filtrate, and obtain.
[0052] (2) Reference substance solution: take about 10mg of ezetimibe reference substance and about 10mg (specification: 10mg / 10mg) or 5mg (specification: 10mg / 5mg) of rosuvastatin calcium reference substance, weigh them accurately, and place in 100ml volume In the bottle, add water-acetonitrile-acetic acid (40:60:0.1) to dilute to the mark, and shake well. Precisely measure 1ml, put it in a 100ml measuring bottle, add water-acetonitrile-acetic acid (40:60:0.1) to dilute to the mark, and shake well.
[0053] (3) Brand model of high perfo...
Embodiment 3
[0064] (1) Sample preparation: take 5 tablets of ezetimibe rosuvastatin calcium tablets, put them in a 50ml measuring bottle, add 12ml of water-acetonitrile-acetic acid (40:60:0.1), ultrasonicate for 30min, cool to room temperature, add water - Dilute acetonitrile-acetic acid (40:60:0.1) to the mark, centrifuge for 5 minutes (10000 rpm), take the supernatant, filter it with a 0.45 μm PP filter head, discard 1 ml of the filtrate, and obtain.
[0065] (2) Reference substance solution: take about 10mg of ezetimibe reference substance and about 10mg (specification: 10mg / 10mg) or 5mg (specification: 10mg / 5mg) of rosuvastatin calcium reference substance, weigh them accurately, and place in 100ml volume In the bottle, add water-acetonitrile-acetic acid (40:60:0.1) to dilute to the mark, and shake well. Precisely measure 1ml, put it in a 100ml measuring bottle, add water-acetonitrile-acetic acid (40:60:0.1) to dilute to the mark, and shake well.
[0066] (3) Brand model of high perfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com