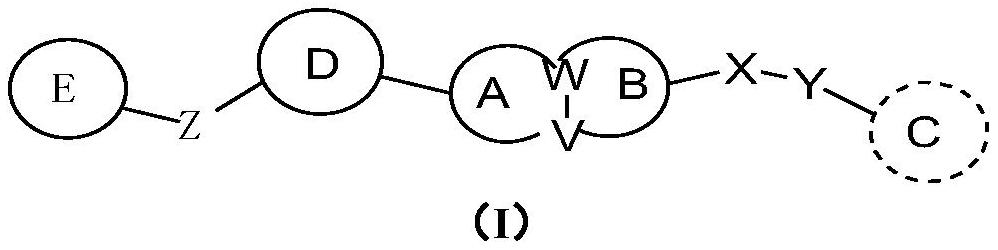
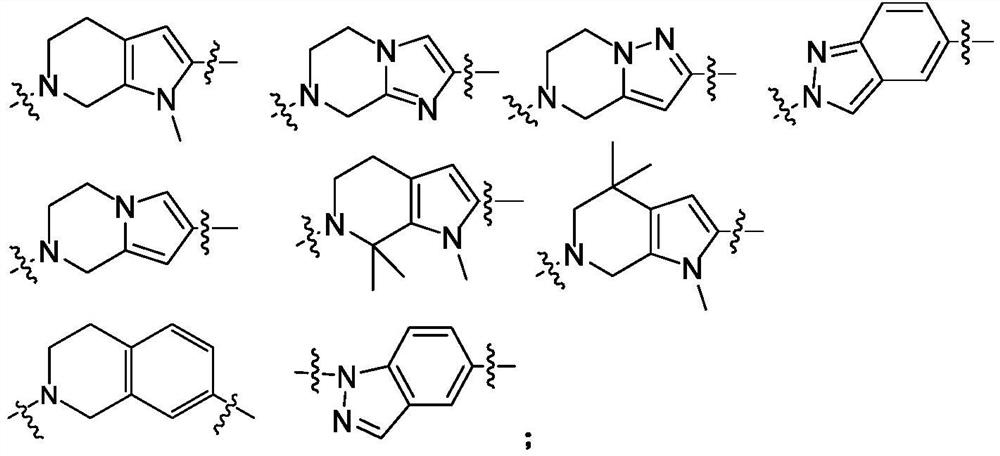
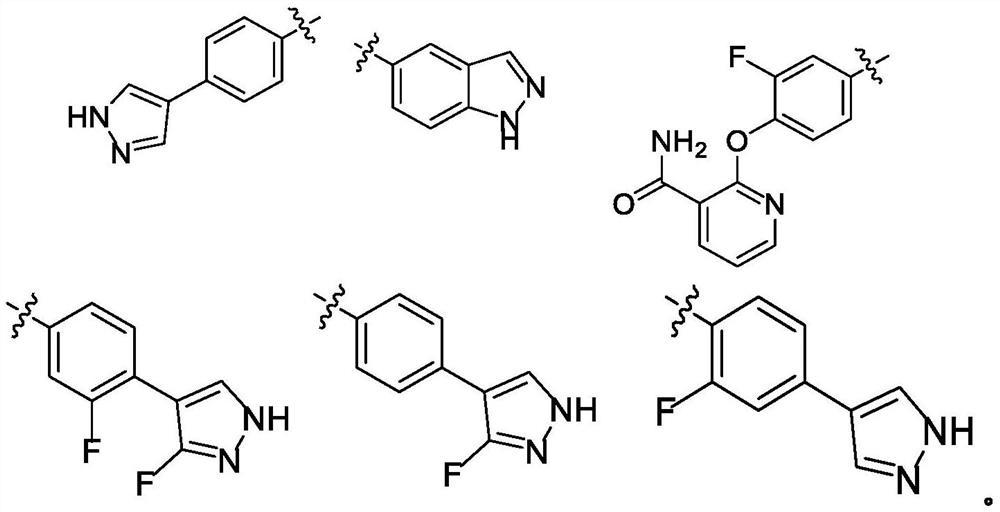
ROCK inhibitor as well as preparation method and application thereof
A technology of solvates and compounds, which is applied in the field of ROCK inhibitors and their preparation, achieves good application prospects, simple preparation methods, good safety and metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0266] Example 1, Compound 1 6-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)pyrimidin-2-yl)-1-methyl-4,5,6,7- Preparation of tetrahydro-1H-pyrrolo[2,3-c]pyridine-2-carboxylic acid (L001)
[0267]
[0268] 1.1 Preparation of compound 3-(3-nitropyridin-4-yl)-2-carbonyl propionate ethyl ester (L001-1)
[0269] Under nitrogen protection, 4-methyl-3-nitropyridine (10.0 g, 72.0 mmol) was dissolved in diethyl oxalate (50 mL), DBU (12.7 g, 83.0 mmol) was added to the reaction solution, and stirred overnight at room temperature. Add ice water (30.0g) to the reaction system to quench, adjust the pH to 4-5 with 1N dilute hydrochloric acid, filter, wash the filter cake with ethyl acetate (100ml*2), and obtain 18.0g red solid after oven drying. The crude product is cast directly to the next step. LC-MS[M+H] + :239.1.
[0270] 1.2 Preparation of compound 1H-pyrrolo[2,3-c]pyridine-2-carboxylic acid ethyl ester (L001-2)
[0271] Hydrogen protection, 3-(3-nitropyridin-4-yl)-2-carbonylpropanoic...
Embodiment 2
[0286] Example 2, compound 7-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)pyrimidin-2-yl)-5,6,7,8-tetrahydroimidazo[1 ,2-a] Preparation of pyrazine-2-carboxylic acid (L002)
[0287]
[0288] 2.1 Preparation of compound imidazo[1,2-a]pyrazine-2-carboxylate ethyl ester (L002-1)
[0289] Pyrazin-2-amine (3.5 g, 36.82 mmol) and ethyl 3-bromo-2-carbonylpropionate (7.0 g, 44.18 mmol) were dissolved in ethylene glycol dimethyl ether (60 mL), and heated at 60° C. overnight. Cool to room temperature, collect the filter cake by filtration, add ethanol (40 mL), and heat at 80° C. for 3 hours. After concentration under reduced pressure, saturated sodium bicarbonate solution (30 mL) was added, extracted with ethyl acetate (30 mL*3), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified on a silica gel column (dichloromethane:ethyl acetate=1:1) to obtain 1.15 g of a yellow oily product with a yield of 16.4...
Embodiment 3
[0296] Example 3, compound (5-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)pyrimidin-2-yl)-4,5,6,7-tetrahydropyrazolo Preparation of [1,5-a]pyrazin-2-yl)(3,3-difluoroazetidin-1-yl)methanone (L003)
[0297]
[0298] 3.1 Preparation of compound 1-(2-((tert-butoxycarbonyl)amino)ethyl)-1H-pyrazole-3,5-dicarboxylic acid dimethyl ester (L003-1)
[0299] Add dimethyl 1H-pyrazole-3,5-dicarboxylate (5.00g, 27.10mmol) into anhydrous N,N-dimethylformamide (60mL), add potassium carbonate (5.61g, 40.60mmol) , ice-water bath, nitrogen protection, and then added tert-butyl (2-bromoethyl)carbamate (6.64g, 29.80mmol) to it, and stirred at room temperature for 3h. Add water (100 mL) to dilute, a large amount of solid precipitates out, filter with suction, and wash the filter cake with water (50 mL). The solid was collected and dried to obtain 8.70 g of crude product with a purity of 70% and a white solid with a yield of 68%. MS[M+H] + =350.2.
[0300] 3.2 Preparation of compound 1-(2-((tert-but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com