Ultra-violet absorbing compounds
A compound and ultraviolet technology, applied in the direction of cosmetics, cosmetic preparations, chemical instruments and methods, etc., can solve problems such as no observed characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] Now, preferred embodiments of the present application will be described in detail with reference to the accompanying drawings. However, they are not intended to limit the scope of the application.
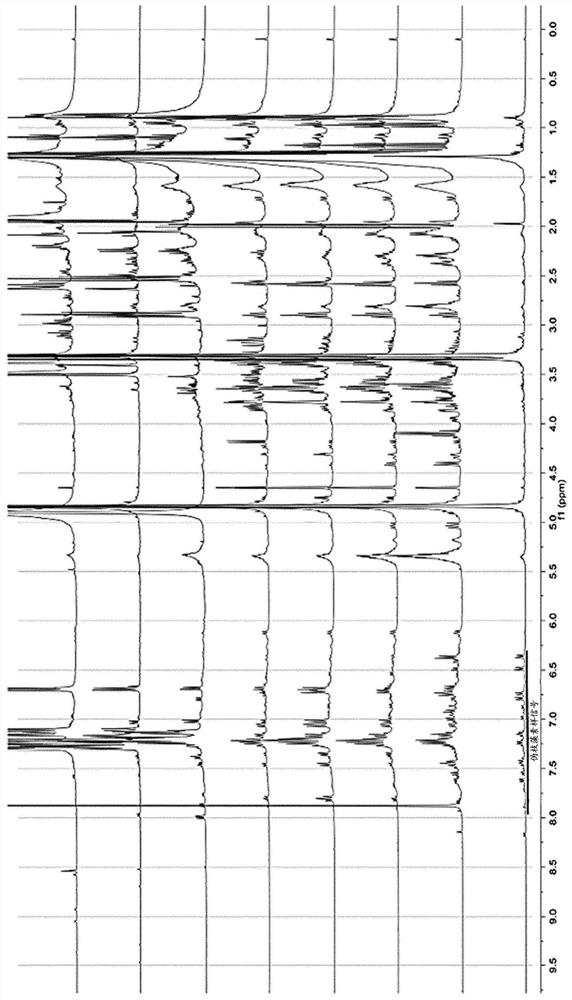
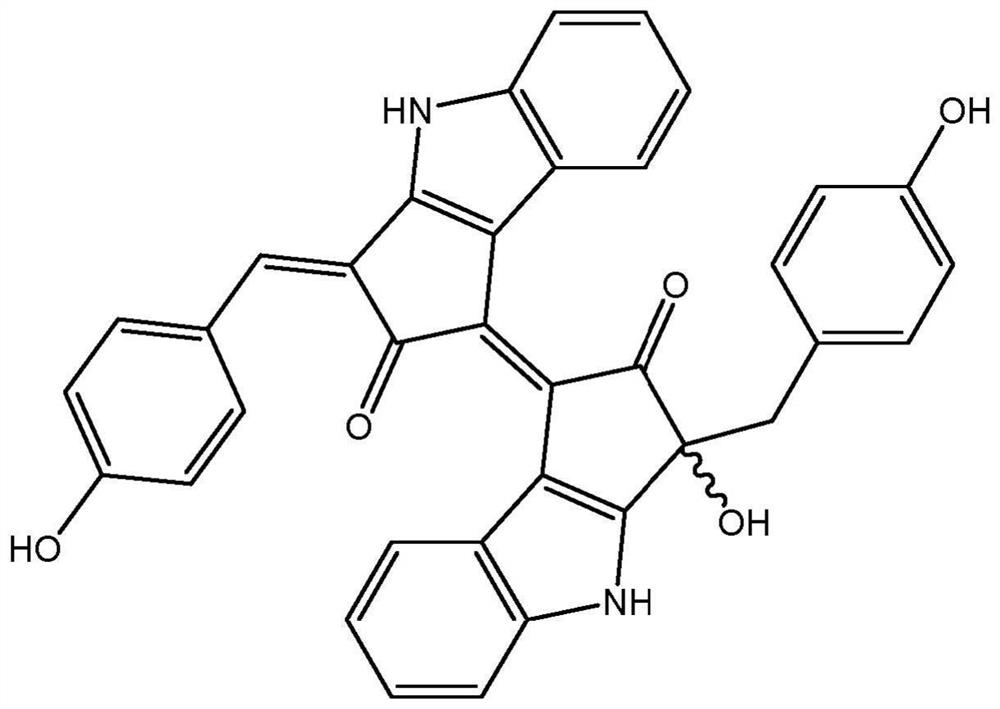
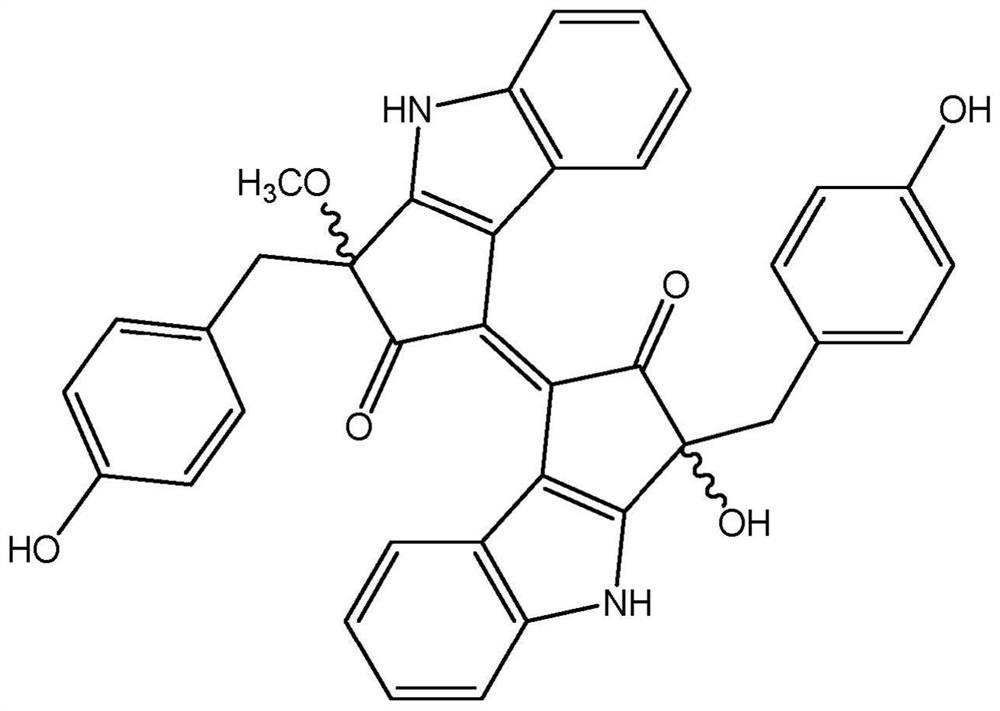
[0048] The present application relates to pseudocladin analogs (scytoneman-like compounds). The compounds were isolated from cyanobacteria collected from a small salt pond located behind the main sand dunes of Mrizika, Morocco (GPS coordinates: 32.955807, -8.779638). Morphological analysis allowed the classification of dominant cyanobacteria as members of the genus Lyngbya. To explore the chemical diversity of mats, biomass was freeze-dried (650 g, d.w.) and extracted using a dichloromethane / methanol mixture (2:1). The crude extract was then fractionated by vacuum liquid chromatography using a solvent gradient of increasing polarity from 100% hexane to 100% ethyl acetate and then to 100% MeOH. Fractions containing pseudocladin-like signals in 1H NMR analysis were pooled a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com