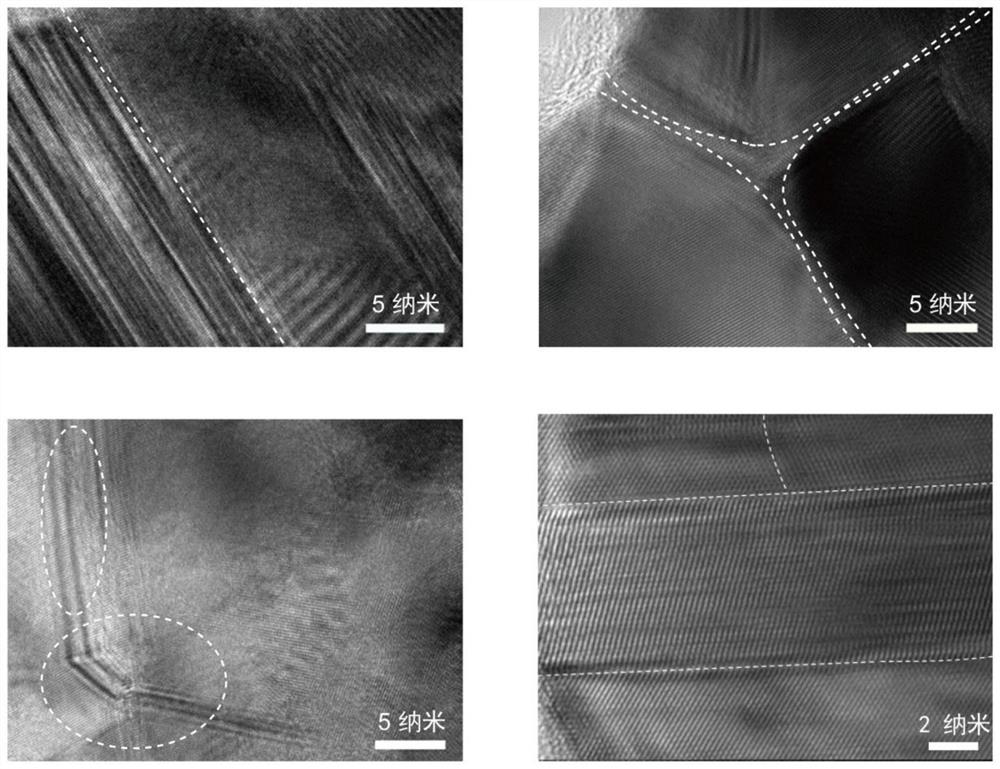
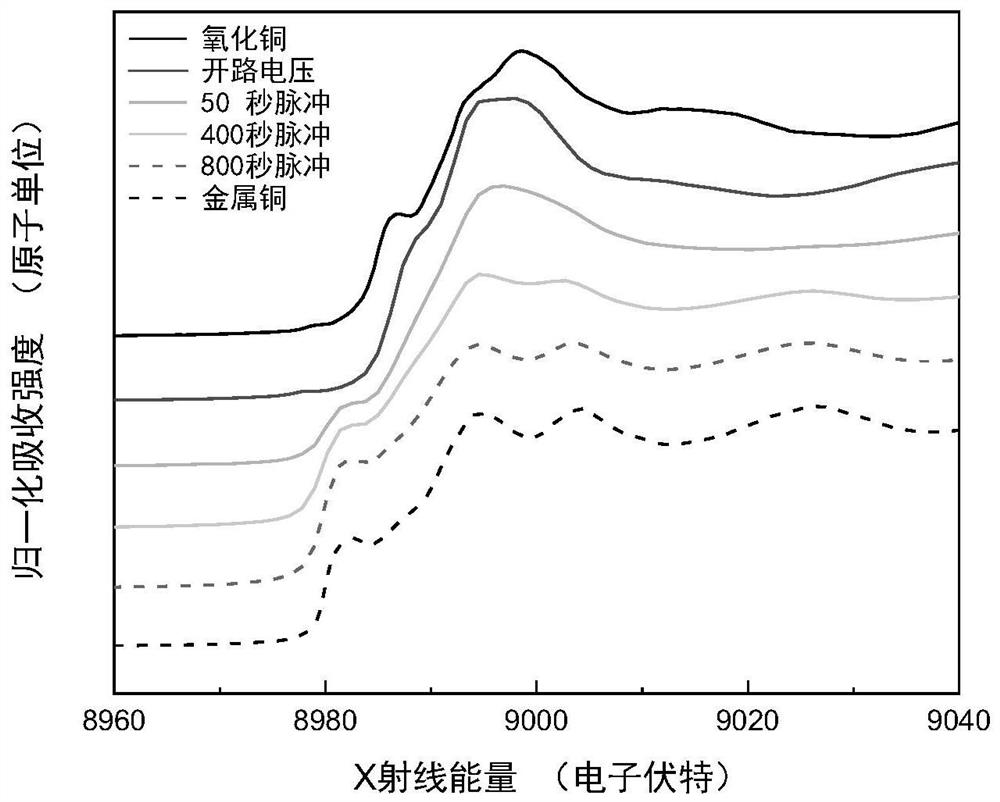
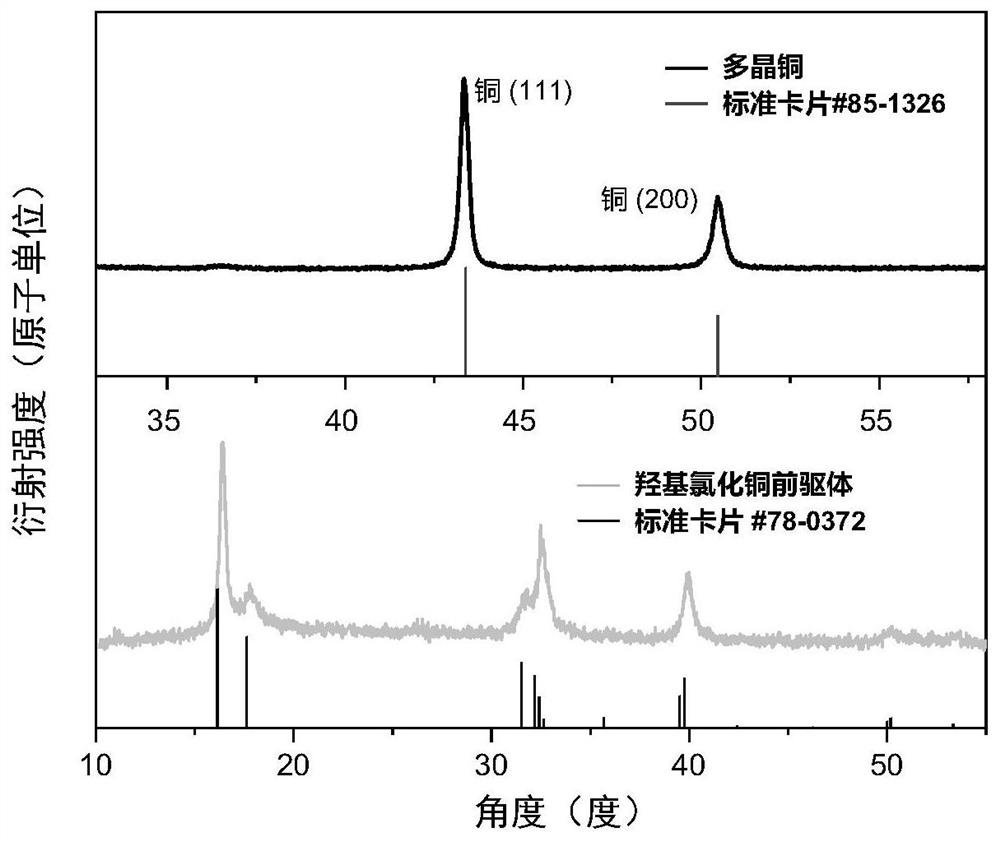
Polycrystalline copper nano material, and preparation method and application thereof
A nanomaterial and polycrystalline copper technology, applied in the field of polycrystalline copper nanomaterials and their preparation, can solve problems such as increased production cost and achieve the effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] 1. Preparation of copper hydroxychloride precursor:
[0090] Dissolve 511mg of copper chloride dihydrate in 2mL of isopropanol to form a green solution. After the solid is completely dissolved, add 2mL of propylene oxide to the mixed solution to disperse evenly by ultrasonication. Then 0.2 mL of water was added to the above solution to disperse evenly, and then the aging step was continued at room temperature for 12 hours. After aging, the supernatant was removed, the solid product was washed with acetone, washed three times and then transferred to a vacuum oven for drying. After drying at room temperature for 24 hours, it was taken out and ground with a mortar to obtain a copper hydroxychloride precursor.
[0091] 2. Electrochemical in situ synthesis of polycrystalline copper nanomaterials
[0092] Take 10 mg of powdered precursor and dissolve it into a mixed solution composed of 0.5 mL ethanol and 0.5 mL water, add 50 μL of 5 wt% Nafion117 solution, and mix in an ult...
Embodiment 2
[0098] 1. Preparation of copper hydroxychloride precursor:
[0099] Dissolve 511mg of copper chloride dihydrate in 2mL of isopropanol to form a green solution. After the solid is completely dissolved, add 2mL of propylene oxide to the mixed solution to disperse evenly by ultrasonication. Then 0.2 mL of water was added to the above solution to disperse evenly, and then the aging step was continued at room temperature for 24 hours. After aging, the supernatant was removed, the solid product was washed with acetone, washed three times and then transferred to a vacuum oven for drying. After drying at room temperature for 24 hours, it was taken out and ground with a mortar to obtain a copper hydroxychloride precursor.
[0100] 2. Electrochemical in situ synthesis of polycrystalline copper nanomaterials
[0101] Take 10 mg of powdered precursor and dissolve it into a mixed solution composed of 0.5 mL ethanol and 0.5 mL water, add 50 μL of 5 wt% Nafion117 solution, and mix in an ult...
Embodiment 3
[0103] Catalytic performance test of polycrystalline nano-copper materials
[0104] The catalytic performance test of the carbon monoxide electroreduction reaction using the polycrystalline copper nanomaterial prepared in Example 1 of the present invention
[0105] 12 mg of the polycrystalline copper nanocatalyst prepared in Example 1 was mixed with 10 μL of perfluorosulfonic acid resin and 1 mL of isopropanol by ultrasonic, and then coated on a 2 cm × 2 cm carbon gas diffusion layer (GDL), and cut out 1 cm after drying. The carbon paper of × 2cm is as working electrode and with the potassium hydroxide (KOH) solution of 1M as electrolytic solution, measure the activity of the polycrystalline copper nano material that embodiment 1 makes as carbon monoxide electroreduction catalyst in flow electrolytic cell, in The electroreduction performance of carbon monoxide was tested in a flow electrolytic cell. The flow electrolytic cell used used a three-electrode system. The carbon pape...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com