Application of aesculin XVII or pharmaceutically acceptable salt thereof in preparation of medicine for preventing and treating non-alcoholic fatty liver disease
A technology for fatty liver disease and escin, applied in the field of biomedicine, can solve problems such as unclear efficacy, achieve the effects of reducing lipid accumulation, improving NAFLD, and increasing ALB content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Study on the effect of the concentration of escin XVII on the viability of HepG2 cells
[0033] 1. Experimental reagents
[0034] Aescin XVII was purchased from Chengdu Desite Biotechnology Co., Ltd. (purity ≥ 98%), HepG2 cells were purchased from American Tissue Culture Collection (ATCC, Manassas, VA, USA), penicillin and streptomycin were purchased from Sigma ( St.Louis, MO, USA), EMEM medium was purchased from Gibco, Invitrogen Corporation (NY, USA), and CCK8 kit was purchased from Beyond Biotechnology Co., Ltd. (ShangHai, China).
[0035] 2. Cell culture and cell viability assay
[0036] Experimental group: HepG2 cells were incubated with escin XVII at final concentrations of 0.1, 0.3, 1, 3, 10, and 30 μg / ml in the culture medium;
[0037] Control group: culture medium without escin XVII to incubate HepG2 cells.
[0038] The HepG2 cells were subcultured in the EMEM medium containing penicillin (final concentration of 100 U / ml), streptomycin (final conce...
Embodiment 2
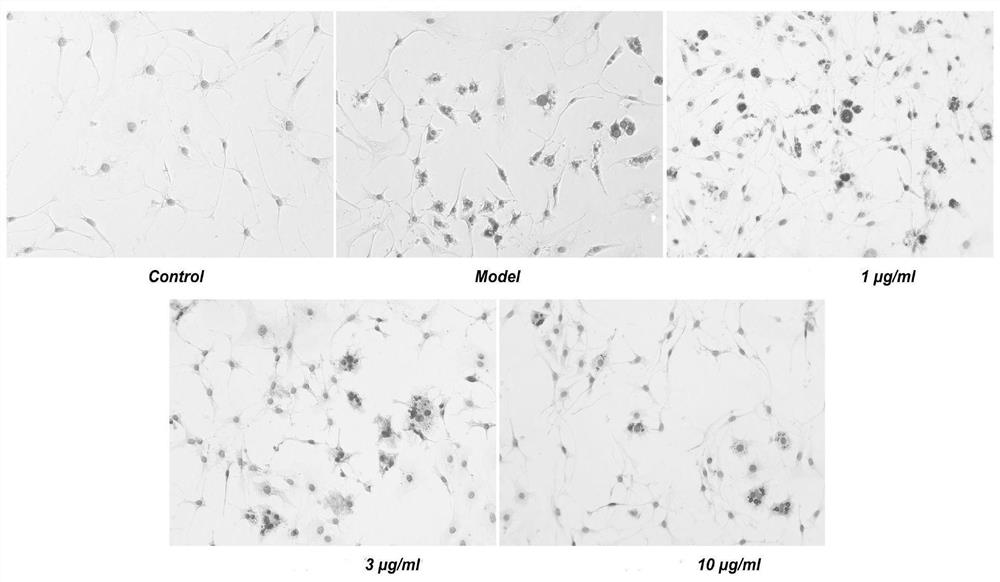
[0042] Example 2 Study on the therapeutic effect of escin XVII on NAFLD
[0043] 1. Experimental reagents
[0044] FBS was purchased from Gibco, Invitrogen Corporation (NY, USA); trypsin was purchased from Gibco (Grand Island, NY, USA); penicillin and streptomycin were purchased from Sigma (St.Louis, MO, USA); EMEM medium was purchased from Gibco, Invitrogen Corporation (NY, USA); Oil Red O staining solution was purchased from Suo Laibao Company (Beijing, China); Triglyceride Assay Kit-Quantification (ab65336) was purchased from Abcam Company (Cambridge, UK); ASTActivity Assay Kit (K753 -100), ALT Activity Assay Kit (K752-100) were purchased from BioVision Company (Milpitas, CA, USA); Human Albumin ELISA Kit (SEKH-0081) was purchased from Suleibao Company (Beijing, China); Human TNF-alpha Quantikine ELISA Kit (DTA00D), Human IL-1β / IL-1F2 Quantikine ELISA Kit (DLB50) were purchased from R&D Company (Minnesota, USA).
[0045] 2. Cell culture and determination of related parame...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com