Inhibiting cyclic amp-responsive element-binding protein (CREB)
A compound and composition technology applied in the field of cyclic AMP-responsive element binding proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
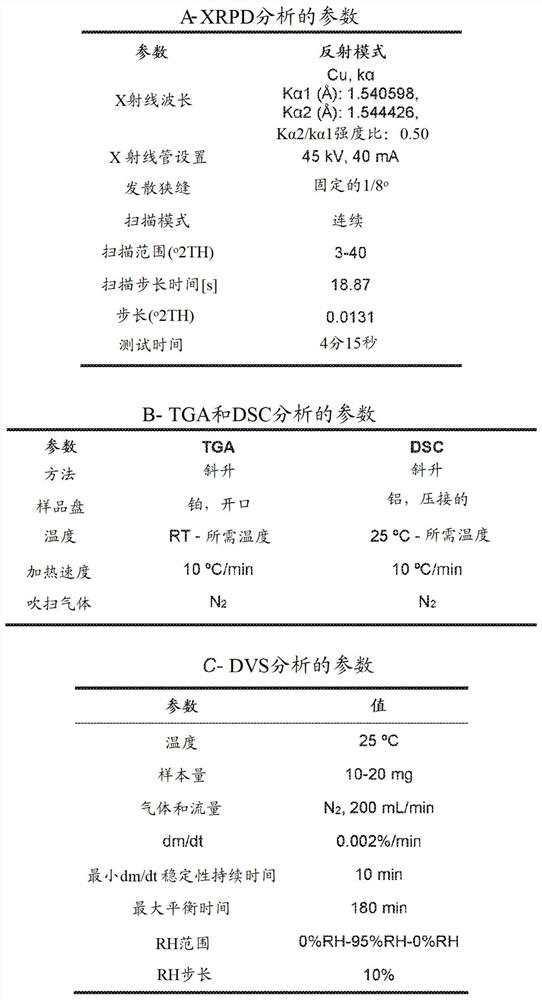
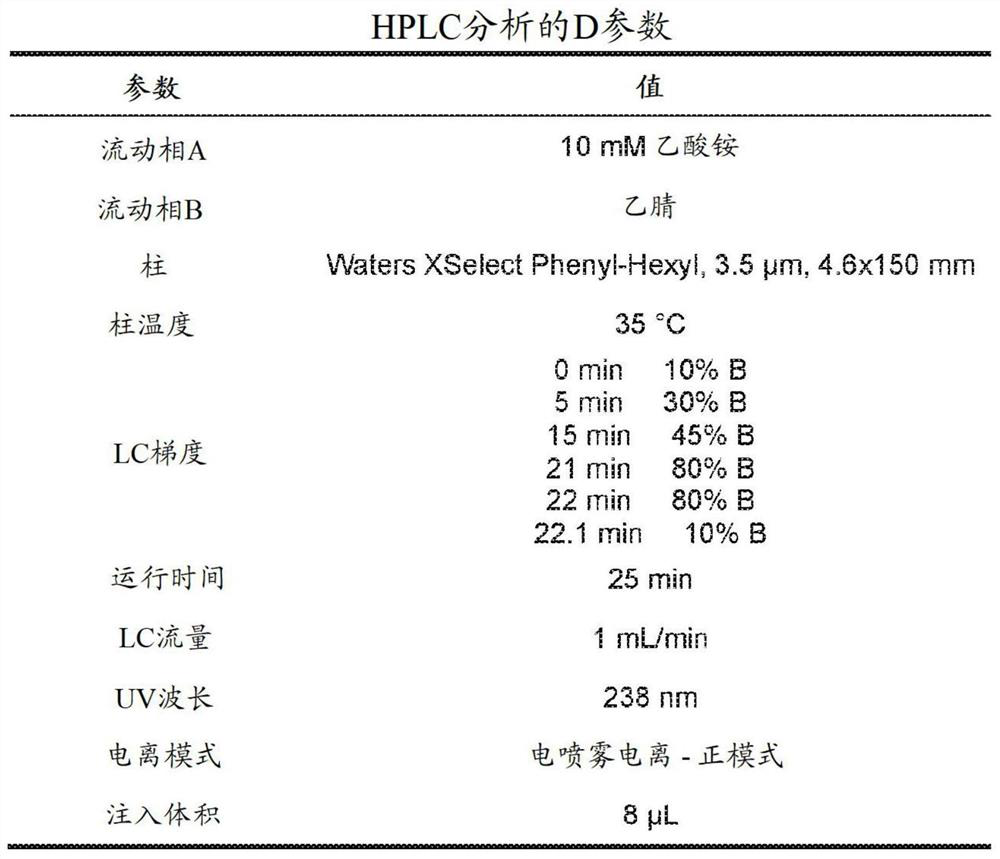
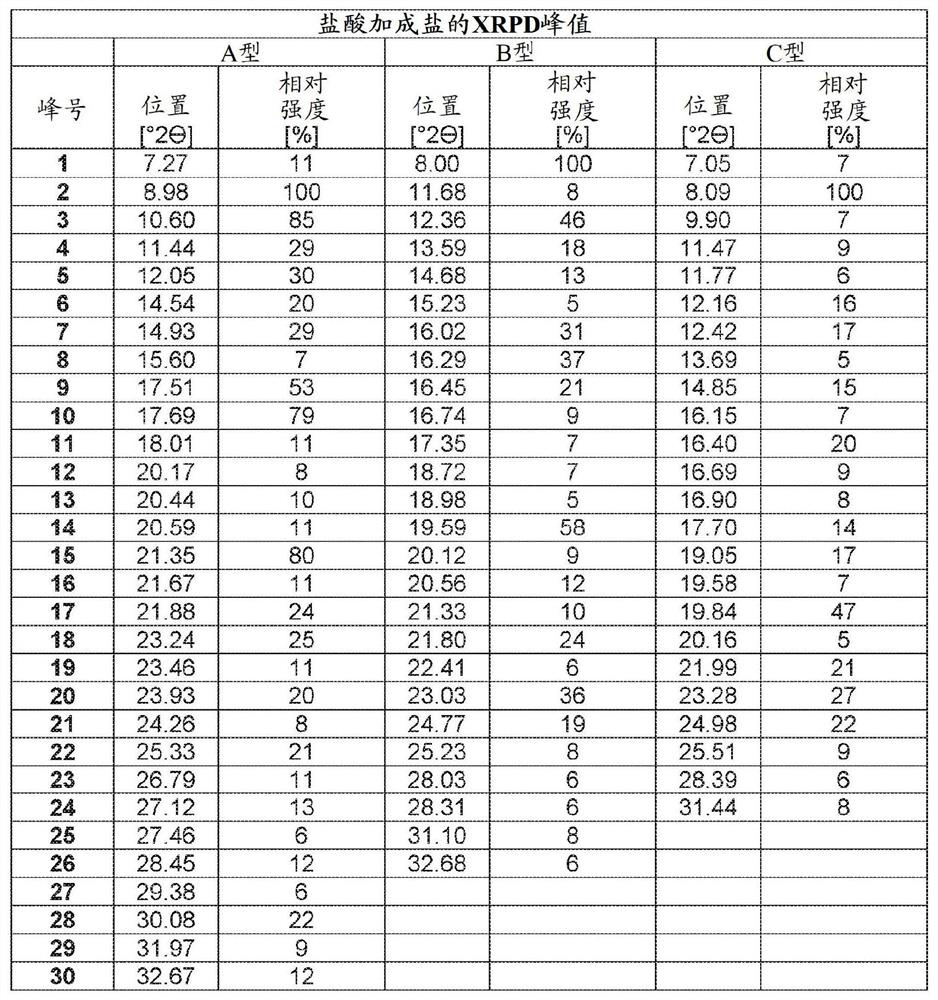
[0143] Example 1 - Preparation of Compounds, Solid Forms and Salts
[0144] Example 1.a - Preparation of Amorphous Free Base + Form
[0145] (1R,3R)-3-[(7S)-2-[(R)-(5-fluoro-2-methoxyphenyl)(hydroxy)methyl]-6-(methoxycarbonyl)-7 -Methyl-3H,6H,7H,8H,9H-imidazo[4,5-f]quinolin-3-yl]cyclohexane-1-carboxylic acid (1);
[0146] (1R,3R)-3-[(7S)-2-[(S)-(5-fluoro-2-methoxyphenyl)(hydroxy)methyl]-6-(methoxycarbonyl)-7 -Methyl-3H,6H,7H,8H,9H-imidazo[4,5-f]quinolin-3-yl]cyclohexane-1-carboxylic acid (1')
[0147] Synthesis of intermediate 2-(5-fluoro-2-methoxyphenyl)-2-hydroxyacetic acid
[0148]
[0149] Step 1. 2-(5-Fluoro-2-methoxyphenyl)-2-[(trimethylsilyl)oxy]acetonitrile
[0150] ZnI 2 (1.6mg, 0.01mmol, a solution of 5-fluoro-2-methoxybenzaldehyde (1.54g, 9.99mmol) in trimethylsilanecarbonitrile (1.5mL, 11.25mmol) was stirred for 1 hour. The resulting mixture was Concentration under vacuum. The resulting crude product was purified by silica gel chromatography (eluting wit...
Embodiment 2
[0187] Example 2 - Kinetic Solubility Evaluation
[0188] The solubility of Form A HCl salt, Form B tosylate salt, and Form A free form was measured in water, SGF, FaSSIF and FeSSIF at 37°C with a solids loading of approximately 10 mg / mL (calculated as free base). All solubility samples were kept rotating at 25 rpm at 37°C and samples were taken at 1 h, 4 h and 24 h. The supernatant was extracted by centrifugation before filtration and used for solubility and pH measurements. The residual solid was collected for XRPD characterization. The results are summarized in Figure 7 middle.
Embodiment 3
[0189] Example 3 - HTRF Biochemical Assay of CBP and BRD4 Activity
[0190] The ability of amorphous Compound 1 to selectively inhibit CBP was determined using the following HTRF biochemical assays of CBP and BRD4 activity. Compound 2 was used as a reference compound:
[0191]
[0192] The assay was performed in an assay buffer containing 50 mM Hepes (pH 7.5, (0.5M Hepes, pH 7.5 solution; Teknova H1575)), 0.5 mM GSH, 0.01% BGG (0.22 μM filtered, Sigma, G7516-25G), 0.005% BSA (0.22 μM filtered, EMD Millipore Corporation, 126575) and 0.01% Triton X-100 (Sigma, T9284-10L). Nanoliter volumes of 10-point, 3-fold serial dilutions in DMSO were pre-distributed into 1536 assay plates (Corning, #3724BC) at final test concentrations ranging from 33 μM to 1.7 nM, highest to lowest dose, respectively. 3 μL 2x protein and 3 μL 2x peptide ligand were added to assay plates (pre-imprinted with compounds). Plates were incubated at room temperature for various times before measuring the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com